Granulation and maturation phases of wound healing
The granulation phase
The third phase of wound healing, consisting in the replacement of the provisional fibrin matrix with granulation tissue once the wound has been debrided, includes several sub-phases:
- re-epithelialization
- fibroplasia,
- collagen deposition
- and angiogenesis.
Re-epithelialization
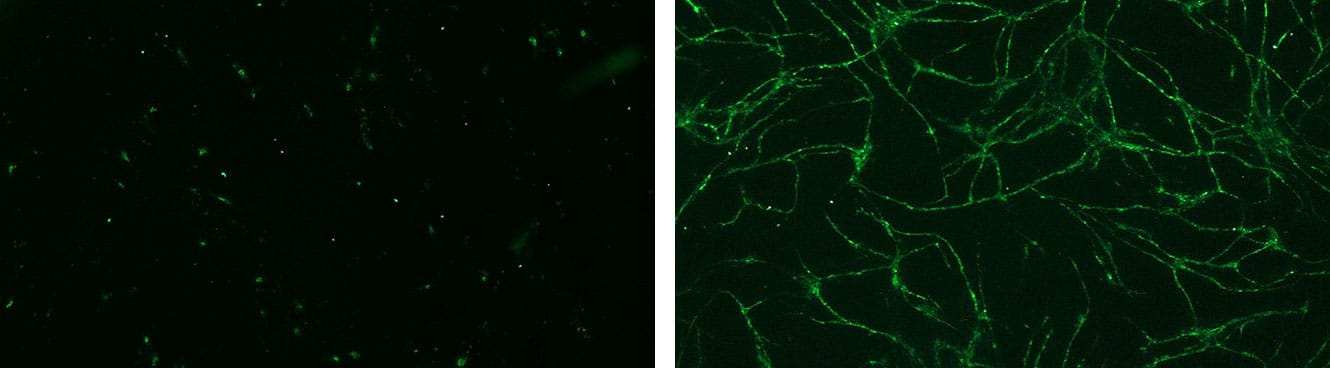
Re-epithelialization, which begins almost in parallel with inflammation, is characterized by the proliferation and migration of epithelial stem cells-derived keratinocytes to the leading edge of the wound [Bioassay: NHEK-0033]. Epithelial cells in dermal appendages undergo epithelial-to-mesenchymal transition to gain motility, then differentiate into keratinocytes expressing specific keratins and other markers such as fillagrin, loricrin and transglutaminase [Bioassays: NHEK-0061 ; NHEK-0062 ; EPIBA-0021 ; EPIBA-0022]. .
Once they encounter the mesenchyme of the ECM, they attach near the inner wound edge and begin to lay down a new basement membrane, leading to the formation of a thin epithelial cell layer, which fills in the defects and protects the wound from infection and desiccation. During this process which is regulated by microRNAs, such as the miR-21, growth factors (EGF, FGFs, TGF-β and interleukins play a key role by stimulating epithelialization. The nervous system seems also important, since sensory neurons accelerate in vitro wound closure by secreting neuropeptides (e.g. substance P, CGRP).
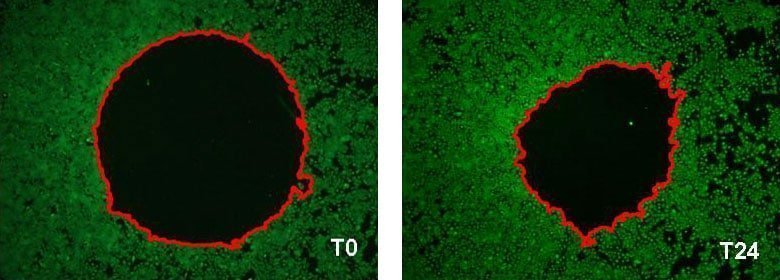
in vitro analysis of NHEK migration by fluorescence imaging based assay.
Example of the wound surface measurement (delimited in red) at T0 and after 24 hours of incubation[Bioassay: NHEK-0033]
Fibroplasia, a key phase of granulation tissue
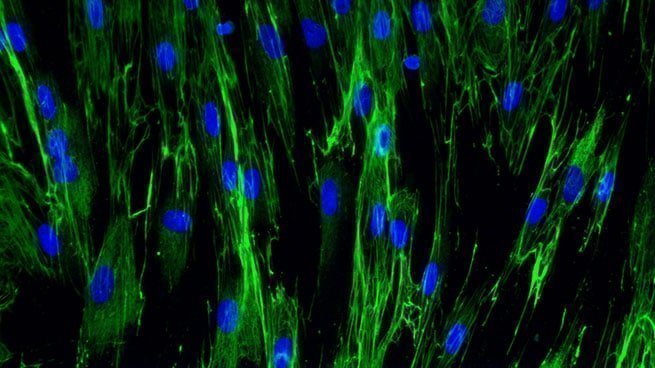
Underneath this new basement membrane, fibroplasia i.e., the proliferation and migration of fibroblasts into the wound, is initiated in response to FGF, TGF-β1 and PDGF [Bioassays: NHDF-0003 ; NHDF-0004]. Fibroblasts differentiate from dermal stem cells and synthesize new ECM components, i.e. type III and type I collagen, glycosaminoglycans, proteoglycans, hyaluronic acid and fibronectin [Bioassays: NHDF-0008 ; NHDF-0009 ; NHDF-0026 ; NHDF-0028 ; NHDF-0030 ; SKIBA-0017]. At the same time, the secretion of MMP decreases and the production of tissue inhibitor of MMP (TIMP) is stimulated to increase the strength of the granulation tissue.
Collagen I expression in normal human dermal fibroblasts
[Bioassay: NHDF-0026]
Collagen III expression in normal human dermal fibroblasts
[Bioassay: NHDF-0028]
Fibronectine expression in normal human dermal fibroblasts
[Bioassay: NHDF-0030]
Angiogenesis
Concurrently to fibroplasia, angiogenesis induced by hypoxia restores vascular perfusion with the formation of new blood vessels. Then platelets-released proangiogenic factors (FGF, PDGF and VEGF) and cytokines stimulate the migration, proliferation and maturation of resident endothelial cells [Bioassay: ENDOF-0001]. In addition, bone marrow-derived endothelial progenitor cells participate in neovasculogenesis at the injury site. LL-37 expressed during the inflammation phase might also be involved in the activation of endothelial cells.
Myofibroblasts and maturation of the granulation tissue
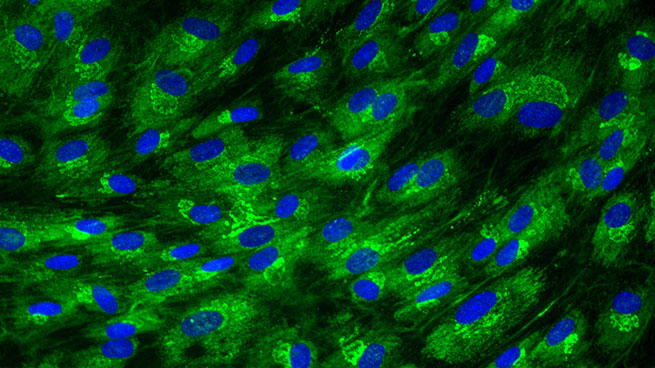
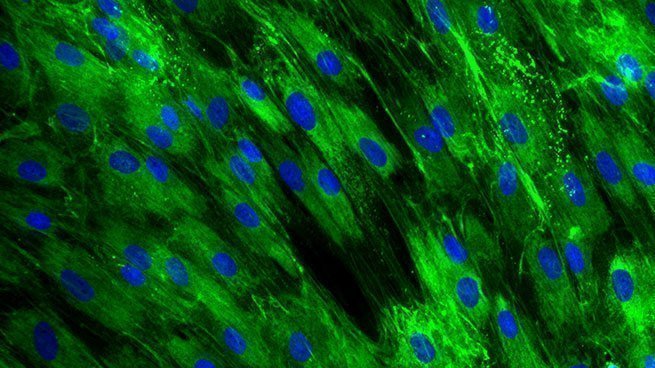
Finally, fibroblasts start to differentiate into myofibroblasts, which acquire contractile properties by expressing alpha-smooth muscle actin in microfilament bundles or stress fibres in response to TGF-β, tissue tension, and matrix proteins, such as ED-A, fibronectin and tenascin C. The contractile forces are then transmitted to the ECM via cytoskeleton-associated and ECM receptor-dependent mechanocoupling focal adhesion complexes, i.e. integrin receptors. Myofibroblasts thus play an essential role in wound contraction, closure of wound edges and maturation of the granulation tissue [Bioassays: NHDF-0035 ; DE-0001; NHDF-0051].
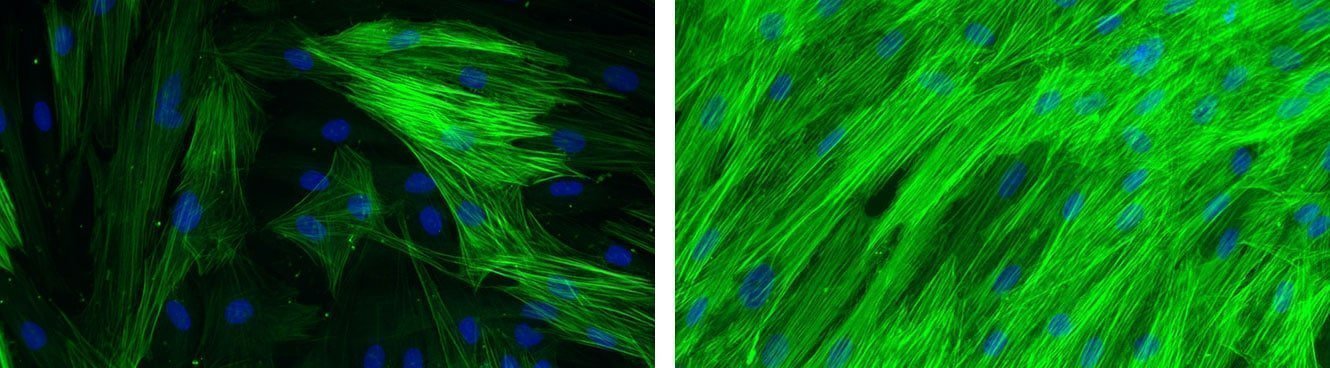
Global effect of VEGF stimulation (right photo) on the formation of pseudotubes in HMVEC/NHDF co-culture.
[Bioassays: ENDOF-0001 et ENDOF-0002]
Global effect of TGF-β stimulation (right photo) on the alpha-SMA expression in normal human dermal fibroblasts.
[Bioassays: NHDF-0035 et NHDF-0051]
The remodeling and maturation phase
During the last remodeling/maturation phase, the wound undergoes progressive and constant remodelling, which can last for years after injury occurred. During this step, which is essential for the restoration of full tissue functionality, MMPs and TIMPs play a prominent role in ECM synthesis and maturation. The collagen bundles increase in diameter and hyaluronic acid and fibronectin are degraded. Progressively, collagen type III, the major component of the granulation tissue, is replaced by collagen type I, the main structural component of the dermis [Bioassays: NHDF-0026 ; NHDF-0011]. This new collagen is more oriented and reorganized into a structurally sound lattice based on glycosaminoglycans and proteoglycans. Elastin, which is absent in the granulation tissue, reappears and contributes to skin elasticity. The final scar reaches a maximum tensile strength of 80% of the original skin and presents a flat and matt aspect with often less pigmentation. The lack of melanin pigments leaves the scar without protection against ultraviolet radiation. Scar repigmentation depends on the depth and width of the wound, and presence of hair follicles containing melanocyte stem cells and melanocyte differentiation and melanogenesis are therefore often disturbed [Bioassay: NHEM-0001].
In the resolution phase of healing, the cell number is dramatically reduced by apoptosis of vascular cells, macrophages and myofibroblasts. Microvascular density also returns to normal by regression of newly formed capillaries. Restoration of skin sensitivity is achieved by the formation of a new sensory neurons network [Bioassay: NS-0001].
Conclusion
Wound regeneration is actually the result of a complex interplay between stem and differentiated skin cells, ECM components (matrix and matricellular proteins) and mechanical forces. Prolongation or deregulation of one or more wound healing phases can cause pathological healing, such as hypertrophic or keloid scars, or chronic wounds. Deciphering the molecular pathways and the role of cells and ECM in normal and pathological wound healing would be of great interest to develop both biomarkers to predict patient healing complications and novel strategies to prevent or treat them (e.g. surgery, therapies and bioengineered products). For instance, the use of stem cells (epithelial, dermal, melanocyte and adipose-derived stem cells) might be relevant in future regenerative cell-based therapies, since they contribute to skin repair throughout wound healing.
Check out Bioalternatives’ updates and experience new testing ideas
- Bioassays, models and services
- Posts and publications
- Events



Wound healing, granulation phase and maturation phase
Wound healingThe third phase of wound healing, consisting in the replacement of the provisional fibrin matrix with granulation tissue once the wound has been debrided, includes several sub-phases: re-epithelialization, fibroplasia, collagen deposition and angiogenesis.
Wound healing, hemostasis phase and inflammatory phase
Wound healingThe inflammatory phase is characterized by the sequential infiltration of polymorphonuclear neutrophils (PMNs), monocytes/macrophages and lymphocytes. IL-8 facilitates PMNs migration from surrounding blood vessels.
Wound healing, overview
Wound healingWound healing is a complex and dynamic process of restoring skin cellular structures and tissue layers that involves multiple components: differentiated cells , stem cells , hair follicles, extracellular matrix (ECM) proteins, cytokines networks, microRNAs , blood vessels, nerves and mechanical forces.
Study of proliferation and 3D epidermal reconstruction from foreskin, auricular and trunk keratinocytes in children
Wound healingOur studies highlight the potential of foreskin tissue for autograft applications in boys. A suitable alternative donor site for autologous cell transplantation in female paediatric burn patients remains an open question in our department. We tested the hypothesis that in vitro studies and RHE reconstructive capacities of cells from different body sites can be helpful to select an optimal site for keratinocyte isolation before considering graft protocols for girls.
Epidermal healing in burns: autologous keratinocyte transplantation as a standard procedure: update and perspective
Wound healingIn the contexte of skin graft, cell suspensions transplanted directly to the wound is an attractive process, removing the need for attachment to a membrane before transfer and avoiding one potential source of inefficiency. Choosing an optimal donor site containing cells with high proliferative capacity is essential for graft success in burns.
Foreskin-isolated keratinocytes provide successful extemporaneous autologous paediatric skin grafts
Wound healingWe report a successful method for grafting paediatric males presenting large severe burns through direct spreading of autologous foreskin keratinocytes. This alternative method is easy to implement, improves the quality of skin and minimizes associated donor site morbidity. in vitro studies have highlighted the potential of foreskin tissue for graft applications and could help in tissue selection with the prospect of grafting burns for girls.
Quantitative and qualitative study in keratinocytes from foreskin in children: Perspective application in paediatric burns
Wound healingKeratinocytes from foreskin have a high capacity for division. A potential source of cells to provide coverage in paediatric burns.
Human embryonic stem-cell derivatives for full reconstruction of the pluristratified epidermis: a preclinical study
Cell and tissue engineering, Dermatology, Pharmacology, Wound healingTo assess whether the keratinocyte progeny of human embryonic stem cells (hESCs) could be used to form a temporary skin substitute for use in patients awaiting autologous grafts, we investigated the cells’ capability of constructing a pluristratified epidermis.
Cultured keratinocyte cells from foreskin and future application for burns in children
Wound healingThe keratinocytes resulting from foreskin have a high capacity of division. These cells can divide a long time before differentiation. The observations enable us to propose with our patients the keratinocytes from foreskin for wound healing especially for burns in children.
Stimulation of the proliferation of human dermal fibroblasts in vitro by a lipidocolloid dressing
Wound healingThe effect of Urgotul on normal human dermal fibroblast proliferation was studied in vitro and compared with that of two other dressing: Mepitel and Tulle Gras.