There are 5 steps for the preparation of samples:
1. Fixation
Fixation is carried out immediately after the removal of the sample to be observed. It is used to immobilize and preserve the sample permanently in as life-like a state as possible. It can be performed using a fixative solution or by freezing:
– Fixation in a fixative solution is carried out by immersing the biological material in a formalin solution.
– Fixation by freezing consists in immersing the sample in a tissue freezing medium (in a liquid state at ambient temperature and in a solid state at -20°C), which is then cooled in liquid nitrogen. The frozen sample is preserved until sectioning. This step is carried out in a mold according to the orientation selected for later sectioning.
2. Embedding
Embedding is the step that follows fixation in a fixative solution. It consists in hardening the sample in a paraffin embedding medium, in order to be able to carry out the sectioning.
It is necessary to dehydrate the sample beforehand, by replacing the water molecules in the sample with ethanol. As ethanol and paraffin are not miscible, an intermediate “clearing” agent that is miscible with paraffin is first used. After the clearing step, the samples are plunged in paraffin baths. These steps are carried out using a dehydration automaton.
The following embedding step is performed by using an embedding center. The sample is placed in a mold filled with molten paraffin and is carefully oriented according to its plane of section. The resulting block is then cooled.
Next the sample is sectioned using a microtome.
3. Sectioning
Sectioning is performed using microtomy or cryotomy. Sectioning is an important step for the preparation of slides as it ensures a proper observation of the sample by microscopy.
– Paraffin-embedded samples are cut by cross section, using a microtome, into thin slices of 5 micrometers. Glass slides are covered with a solution that contains an additive in order to keep the section attached onto the slide. Slides are then placed onto a hotplate in order to ensure a uniform spreading of the sample. Once the slides are heated, the residual liquid is removed by hand. The slides are then dried at room temperature.
– Frozen samples are cut using a cryostat. The frozen sections are then placed on a glass slide for storage at -80°C.
Bioalternatives evaluates the suitable fixation and sectioning conditions according to your sample and to your study.
The choice of these preparation conditions is crucial in order to minimize the artifacts. Paraffin embedding is favored for preserving tissues; freezing is more suitable for preserving DNA and RNA and for the labeling of water-soluble elements or of those sensitive to the fixation medium.
4. Staining and immunolabeling
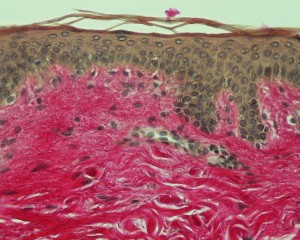
–Staining increases contrasts in order to recognize and differentiate the different components of the biological material.
The sample is first deparaffinized and rehydrated so that polar dyes can impregnate the tissues. The different dyes can thus interact with the components to be stained according to their affinities.
Once staining is completed, the slide is rinsed and dehydrated for the mounting step.
We offer a wide range of routine and special staining techniques . For any special staining request, please contact us.
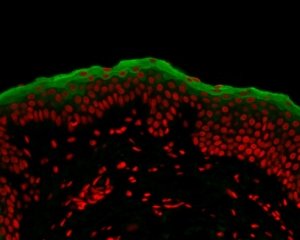
– Immunolabeling detects the particular cell and tissue components (antigens) by using polyclonal or monoclonal antibodies.
A solution, which contains the antibody, is deposited onto the sample, which is then washed in order to remove the unbound antibodies.
Our team can use either the direct or the indirect approach for immunolabeling, by using antibodies coupled with fluorochromes (immunofluorescence) or by using enzymes that react to chromogens (immunochemistry)
Our histology laboratory is here to assist you with any request concerning immunolabeling, search and validation of antibody and for colabeling.
5. Mounting
Sections are mounted between slides and coverslips using a product to ensure adhesion. The slides are then ready for storage or observation.
For fluorescence microscopy observations, a mounting medium is used in order to temporarily decrease the fluorescence loss.