Related Publications
SELECTED PUBLICATIONS ON VITILIGO
>> Check the full list HERE.
Conference Contributions
We share our research on dermatology and trichology through international conferences and publications, contributing to the advancement of research in these fields.
>> Check our past contributions HERE.
Preclinical Research Solutions for Vitiligo
IN VITRO MODELS
- NHEK (Normal Human Epidermal Keratinocytes)
- NHEM (Normal Human Epidermal Melanocytes)
- NHEM and NHEM co-culture
- Immune cells from healthy or vitiligo patients
- RHE (Reconstructed Human Epidermis)
- RHEm (RHE containing melanocytes)
EX VIVO MODELS
- Healthy human skin ex vivo
- Experimentally-induced vitiligo-like phenotype in healthy human skin ex vivo
- Vitiligo lesional and perilesional human skin ex vivo
- Fresh whole blood
IN VIVO MODELS
- In vivo humanized mouse model of vitiligo (developed by Prof. Amos Gilhar at the Skin Research Laboratory (Technion-Israel Institute of Technology, Haifa, Israel) without financial support or involvement from QIMA Life Sciences)
We can help you evaluate the following readouts
Melanocyte Number
Melanocyte Dendricity
Melanogenesis
Melanin Quantification
Melanosome Transfer
Melanocyte Apoptosis
Immune System Activation
Immune Cell Number
Cytokine Release
…among many others.
Clinical Research Solutions for Vitiligo
BIOANALYSIS OF CLINICAL SAMPLES
- Non-invasive (swabbing or tape stripping) and invasive (biopsies) sample collection
- Analysis and quantification of cellular components (proteins, lipids) via analytical chemistry
- Vitiligo biomarker analysis in tissue, blood and non-invasive collected samples
CLINICAL TRIALS
- Clinical study implementation
- Clinical study performance
- Data management
- Data analysis
Vitiligo Study Examples
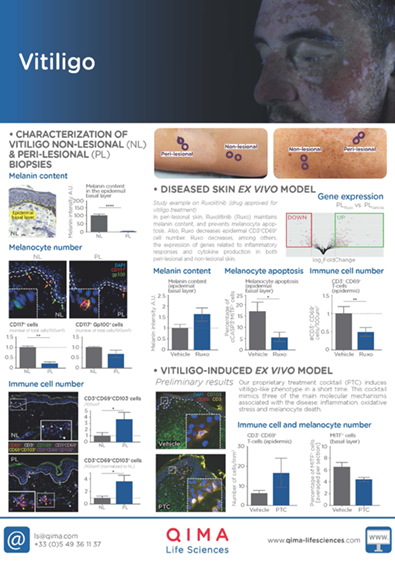
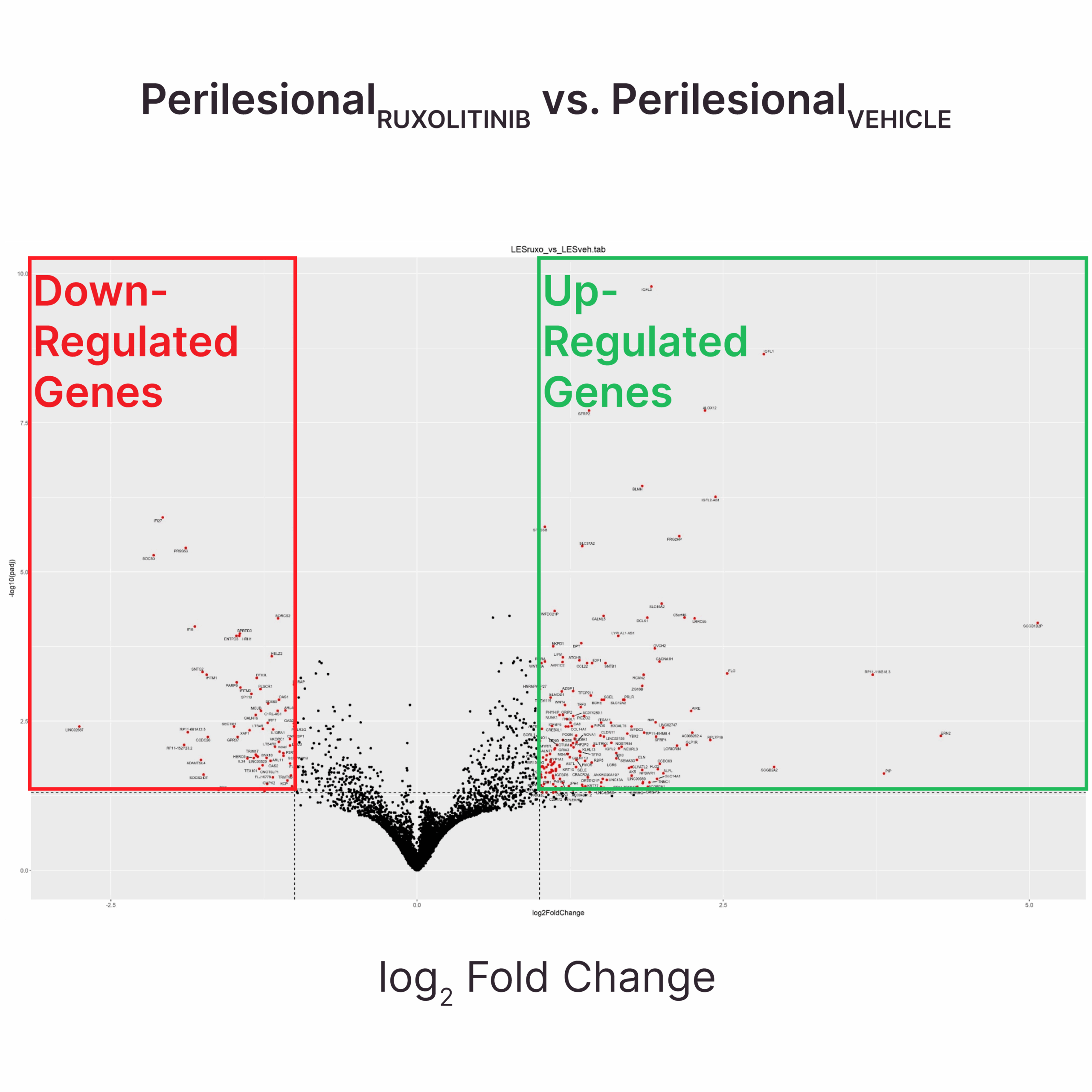
RUXOLITINIB SUPPRESSES TYPE I INTERFERON SIGNALING AND UPREGULATES MELANOCYTE-RELATED GENES IN PERILESIONAL SKIN
Test: Transcriptomic analysis
Method: RNAseq
Model: Diseased skin ex vivo model
Results: Ruxolitinib reduces the expression of genes involved in inflammatory responses and cytokine production in both peri-lesional skin (shown in the figure) and non-lesional skin (data not shown).
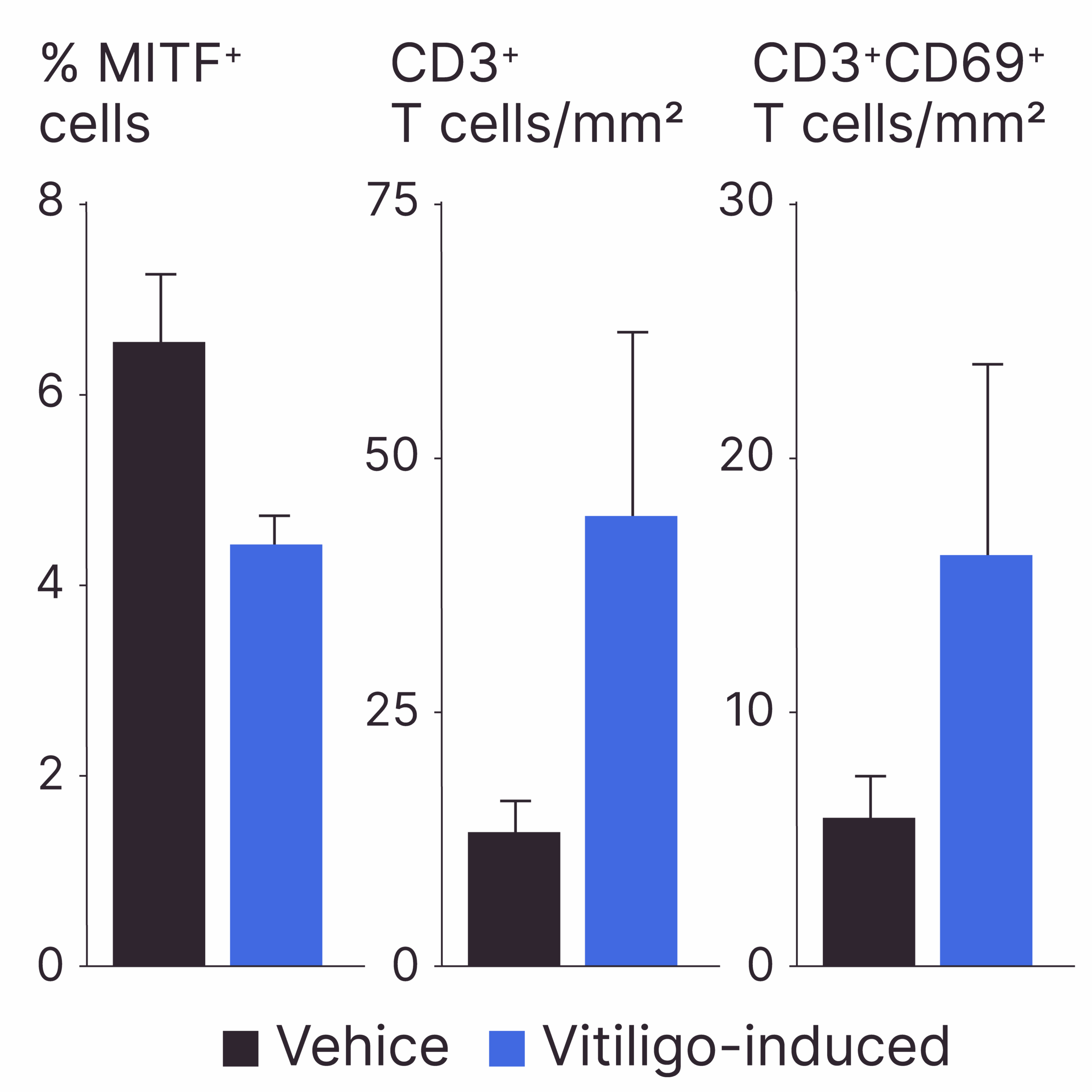
OUR PROPIETARY TREATMENT COCKTAIL INDUCES A VITILIGO-LIKE PHENOTYPE IN A SHORT TIME
Method: Immunohistochemistry
Model: Experimentally-induced vitiligo-like phenotype in healthy human skin ex vivo
Results: Treatment with our propietary cocktail induces three of the main molecular mechanisms associated with vitiligo: inflammation (CD3+ and CD3+69+ cells), oxidative stress (data not shown) and melanocyte death (% MITF+ cells).
XENOTRANSPLANTS IN A HUMANIZED MOUSE MODEL OF VITILIGO RECAPITULATE KEY CLINICAL PHENOTYPES OBSERVED IN HUMAN PATIENTS
Model: In vivo humanized mouse model of vitiligo (developed by Prof. Amos Gilhar at the Skin Research Laboratory (Technion-Israel Institute of Technology, Haifa, Israel) without financial support or involvement from QIMA Life Sciences)
Results: Healthy human skin xenografts, challenged with proprietary stimuli, and injected with sorted CD8+NKG2D+ challenged with proprietary stimuli before and after melanocytic antigen recognition, develop depigmented lesions (shown below). Melanocyte loss, reduced melanin, elevated IFNγ and CXCL10, and immune activation (data not shown) are only some of the key cellular and molecular alterations typical of vitiligo detected in the induced lesions.
AI-BASED TOOL FOR THE EVALUATION OF THE CLINICAL EFFICACY OF VITILIGO TREATMENTS
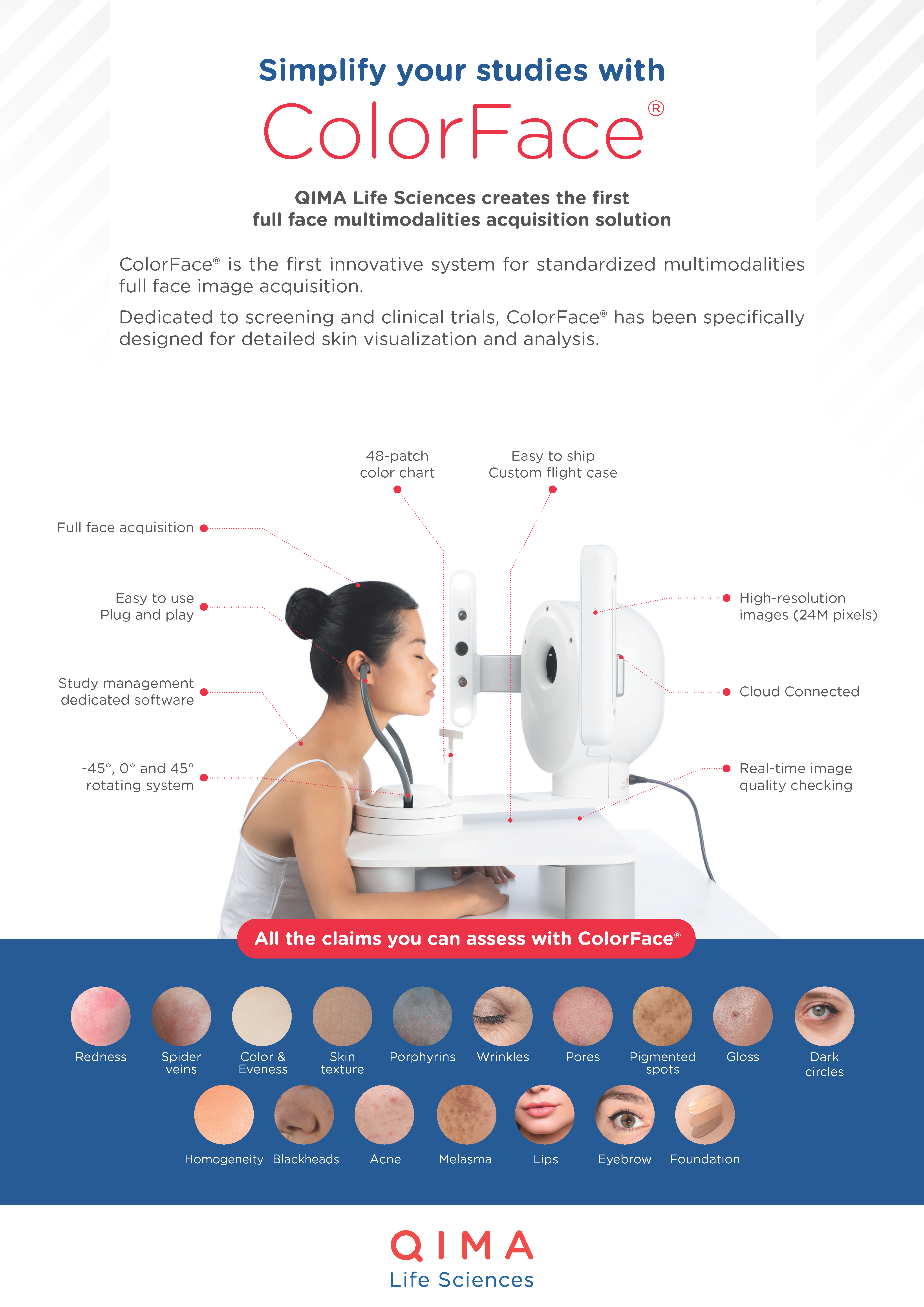
Test: Facial Vitiligo Extent Quantification
Method: Image capture (ColorFace®) and analysis (Vitil-IA®)
Model: Clinical study on vitiligo patients
Results: Assessment over time by Vitil-IA® of the percentage of depigmentation of the face. Week 0 (baseline: depigmentation of 45.5%; Week 12: depigmentation of 20.9%.



At QIMA Life Sciences, we are committed to staying at the forefront of dermatology research by developing innovative approaches.
We offer smart solutions for studying vitiligo using validated models at both preclinical and clinical stages, making us the perfect partner for your research.
Explore Our Models & Assays in Our Webinar
What’s New in Testing?
Blog Articles
PRECLINICAL SOLUTIONS
Vitiligo induced ex vivo model
In vivo humanized mouse model of Vitiligo (developed by Prof. Amos Gilhar at the Skin Research Laboratory (Technion-Israel Institute of Technology, Haifa, Israel) without financial support or involvement from QIMA Life Sciences)
CLINICAL SOLUTIONS
New Solution for Objectively Evaluating the Efficacy of Vitiligo Treatments in Clinical Trials
Interested in Learning More?
Explore Other Related Topics
CLINICAL TRIAL EFFICACY WITH VITIL-IA®

FACE IMAGING SYSTEM: COLORFACE®
VITILIGO