WHAT YOU WILL LEARN IN THIS ARTICLE:
- What Is Inflammation?
- Risks of Excessive Inflammation
- What Causes Cancer?
- What Is the Tumor Microenvironment (TME)?
- Links Between Cancer and Inflammation
- Biomarkers for Cancer
- Anti-Inflammatory Drugs for Cancer
What Is Inflammation?
Inflammation is understood in everyday language as an area of the body that is swollen and often also painful. However, in the field of Immunology, the term “inflammation” takes a very precise meaning.
The term inflammation was initially coined more than 2,000 years ago and was originally defined by the presence of four cardinal signs: redness, swelling, heat, and pain (in Latin: “rubor et tumor cum calore et dolore”). Only by the end of the 18th century inflammation started to be considered as a natural and essential physiological response of the body to aggression (either from infectious or traumatic origin).
Enhanced permeability of blood capillaries enables the recruitment of immune cells to the site of infection or damage, thus explaining both swelling and redness at the inflammation site. Communication among cells is enabled by molecular signals acting as messengers, known as cytokines. Immune cell recruitment is mediated by a specific subtype of cytokines that possess chemotactic activity, and which are therefore known as chemokines.
Discover other blog articles
Risks of Excessive Inflammation
Very early in the study of inflammation, physicians noticed that an excess of inflammation could worsen a patient’s symptoms instead of helping them overcome a disease. Inflammation is therefore often seen as a double-edged sword. It is beneficial only if ordered and timely; otherwise, it can lead to the development of other diseases or even death.
Acute inflammation is characterized by a rapid onset and a short duration whereas chronic inflammation is characterized by a slow onset and longer duration.
Examples of inflammatory conditions include psoriasis, arthritis, and Crohn’s disease. Still, it is also known that several diseases, such as diabetes, atopic dermatitis and Parkinson’s disease, can develop as a result of chronic inflammation.
Examples of acute inflammation include the “cytokine storm” observed for instance in critical patients of COVID-19. Several studies report this excessive cytokine production as responsible for ARDS (acute respiratory distress syndrome) and multiple organ failure, and further show that positive correlates with disease severity.
Inflammation has also been associated with cancer, both as a cause and consequence. The risk of developing cancer is likely to increase upon chronic inflammation of tissues. Contrariwise, precancerous cells produce pro-inflammatory cytokines, which results in inflammation.
You may also be interested in
Immune Response Testing
What Causes Cancer?
Cancer is a disease that develops progressively, i.e., in phases. A single mutagenic event does not result in cancer but can act as initiating event. Cancer initiation can result from activating mutations of oncogenes, deactivating mutations of tumor suppressor genes, or both.
Cancer cells are characterized by the following traits:
- Unrestricted replication: Cancer cells are self-sufficient for growth and insensitive to growth-inhibitory signals.
- Resistance to death, including terminal differentiation program, senescence, or apoptosis.
- Sustained angiogenesis: The creation of new blood vessels helps sustain cancer cells and provide them with the nutrients and oxygen required for survival.
- Ability to invade ectopic tissues
Initially, the Cancerology field held a “cancer-cell-centric” view of cancer, but it was later challenged by the observation that cancer cells actually constitute less than half of the total mass of the tumor. This led scientists to shift towards a more comprehensive analysis: cancer cells must be studied in their biological environment, i.e., the tumor microenvironment (TME).
What Is the Tumor Microenvironment (TME)?
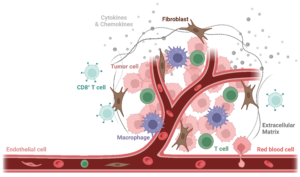
The TME is composed of both cellular and non-cellular components (extra-cellular matrix). Cellular components are highly heterogeneous and include non-malignant stromal cells (mainly endothelial cells and cancer-associated fibroblasts), neoplastic cells (cancer cells), and migratory immune cells. Among the cells of the innate and adaptive immunity are the following: monocytes, myeloid-derived suppressor cells, tumor-associated macrophages (TAMs), tumor-associated dendritic cells (TADCs), CD4+ and CD8+ T cells, Tregs, natural killer cells, etc.
Given the diversity of the cellular components and the variety of their roles, it is easy to see that the cytokine network plays a key role in the TEM. Indeed, the cytokine network orchestrates the recruitment and responses of both cancer and non-malignant host cells.
Figure 1. Scheme of the Tumor Microenvironment. The Tumor Microenvironment (TME) is composed of both cellular and non-cellular components (extracellular matrix, in dark grey). Cellular components are highly heterogeneous and include non-malignant stromal cells (mainly endothelial cells, in light pink, and cancer-associated fibroblasts, in brown), neoplastic cells (cancer cells, in dark pink), and various migratory immune cells, including tumor-associated macrophages (TAMs, in purple) and and CD8+ T cells (in light blue). Image created with Biorender.
Links Between Cancer and Inflammation
A possible link between inflammation and cancer was suggested more than 150 years ago by Rudolf Virchow, who observed the presence of leukocytes within tumors. The German physician suggested at the time that those immune cell infiltrates were at the origin of the tumor; in other words, that cancer originated at sites of chronic inflammation. Yet, the critical role of inflammation in cancer was not proved until recently. Today, inflammation is a well-accepted hallmark of cancer and is associated with high proliferation rates and poor prognosis.
As mentioned above, inflammation can be both a cause or a consequence of cancer.
In the first case, an inflammatory condition precedes the development of malignancy (inflammation promotes or even initiates it). In the second case, the oncogenic changes drive an inflammatory milieu: pro-inflammatory cytokines can be produced by inflammatory cells in the TEM, but also by precancerous cells themselves.
Irrespective of its origin, inflammation contributes to the survival, proliferation, and invasion of malignant cells by subverting the host immune system, stimulating angiogenesis, and altering the response to chemotherapy. Inflammation is also associated with immune suppression, which results in a higher risk of infection for cancer patients.
Paradoxically, although intended to contribute to the host anti-tumor response, inflammation seems more prone to contribute to cancer progression.
“If genetic damage is the “match that lights the fire” of cancer, some types of inflammation may provide the “fuel that feeds the flames”.
(Balkwill F. & Mantovani A., The Lancet, 2001.)
Biomarkers for Cancer
As is the case for other diseases, early cancer detection improves patient outcomes. In this respect, biomarkers are a precious tool not only for cancer diagnosis but also for prognosis and assessment of therapeutic interventions.
A biomarker (or biological marker) is a measurable indicator of a medical state or condition. They can be measured in the body (e.g., blood or soft tissues) or their products (e.g., urine) and serve as indicators of normal or pathological biological processes. The more accurate and reproducible a biomarker, the more reliable the information we can draw from it.
One of the reasons why the link between inflammation and cancer has been of great interest in the cancerology field is precisely the possibility of using establishing biomarkers of inflammation as cancer biomarkers. Cancer biomarkers are very valuable because they inform about a patient’s cancer status and help to stratify patients and adapt treatments. Even more, they usually represent a non-invasive, reliable, and economical approach.
Biomarkers of inflammation can be not only cell effectors (such as immune cells) but also inflammatory molecules (such as cytokines and the acute-phase protein CRP). For instance, biomarkers can be based on a combination of different immune cell counts, such as the systemic immune-inflammation index (SII), which was developed as a prognostic tool and proved successful for different types of cancers. Other examples include biomarker protein panels for cancer detection; briefly, immunoinflammation-related protein patterns are identified through proteomic analysis.
Anti-Inflammatory Drugs for Cancer
Analogously to what occurs with biomarkers, anti-inflammatory drugs have been shown to help cancer prevention and treatment. Therefore, understanding the complex interactions within the TME is critical for developing of novel anti-cancer therapies and optimizing of current therapies.
Among the different targets for cancer therapy, we can mention:
- Signal transducers and transcription factors
- Chemokines and cytokines
- Tumor-promoting immune and inflammatory cells
References
Balkwill F. & Coussens L. M. An inflammatory link. Nature (2004).
Balkwill F. & Mantovani A. Inflammation and cancer: back to Virchow? The Lancet (2001).
Candido J. & Hagemann T. Cancer-Related Inflammation. Journal of Clinical Immunology (2013).
Cavaillon J-M. La Flamme Salvatrice : Il était une fois l’inflammation, Editions DOCIS (2017).
Grivennikov S. I., Greten F. R. & Karin M. Immunity, Inflammation, and Cancer. Cell. 2010.
Watch the Replay of our Webinar on
Immunology Testing
Discover our
oncology-related assays
Find a Research and Testing Program that Suits Your Needs
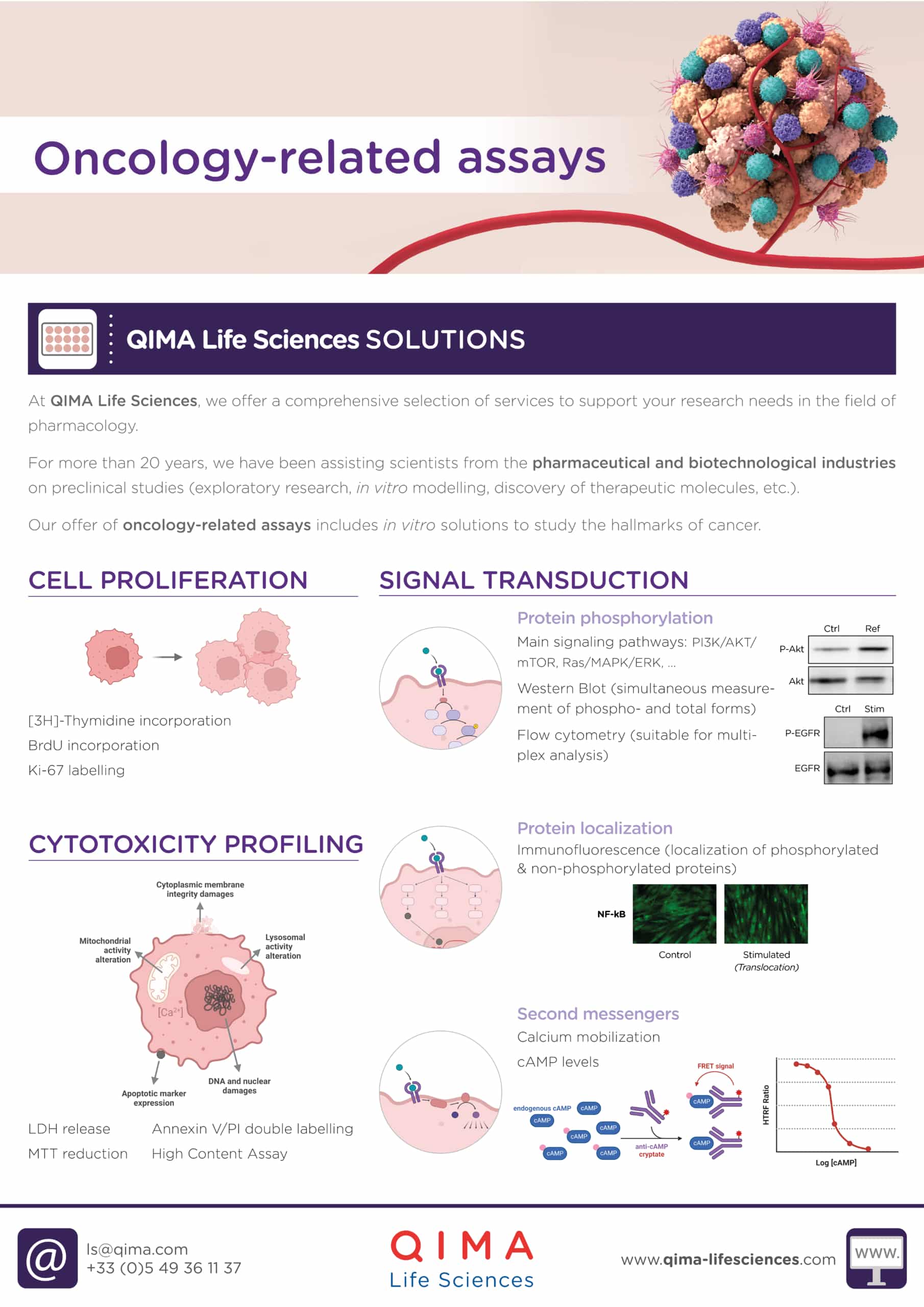
At QIMA Life Sciences, we offer a comprehensive selection of services to support your research needs in pharmacology. Our offer of oncology-related assays includes: in vitro solutions for cytotoxicity profiling, cell proliferation, signal transduction and vascularization assessment, biomarker identification and validation, and immune cells testing.
Our scientists have extensive expertise and know-how that can be applied to a wide range of pre-clinical and clinical research projects. We offer customized technical solutions that can best meet your requirements, prerequisites, and objectives, with particular attention given to the feasibility study and personalized management of your projects. With our state-of-the-art facilities and over 20 years of experience supporting projects in the pharmaceutical and biotechnological industries, we are pleased to offer you dedicated project management support and consulting for your R&D process.
*
Ready to discuss a testing and research program that will suit the needs of your business?