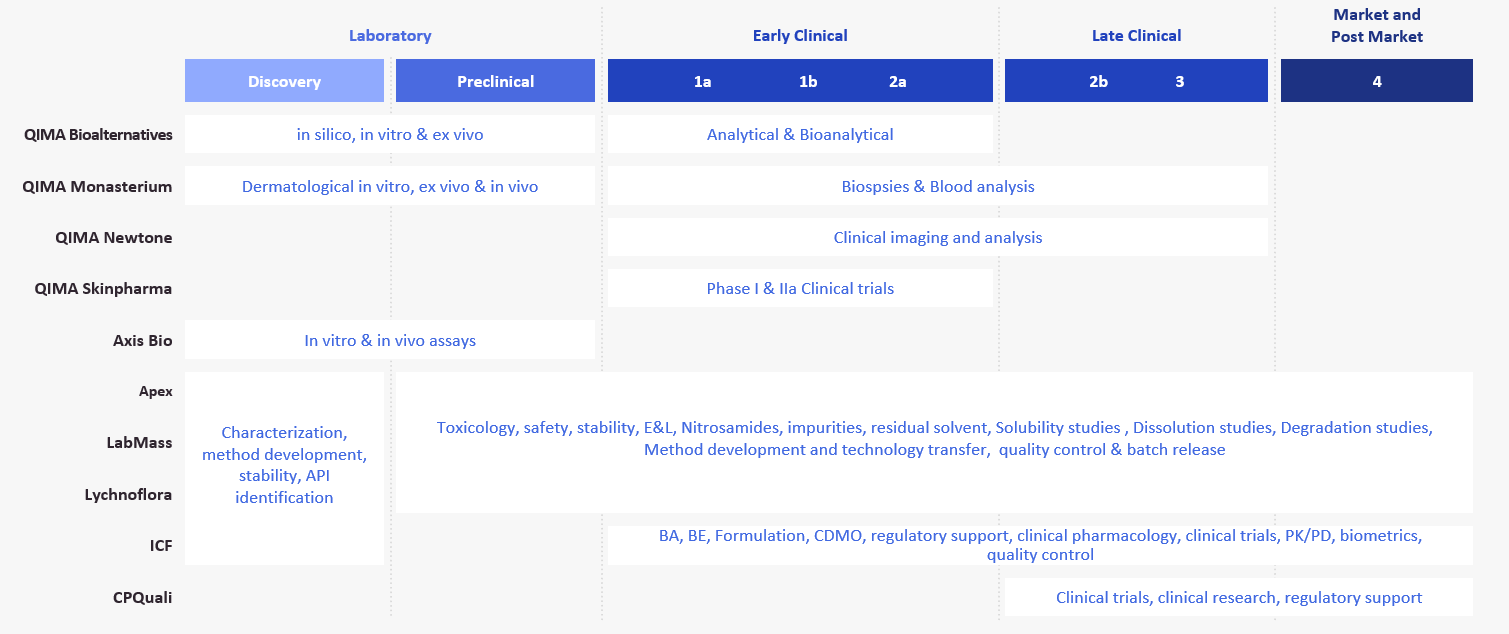
About QIMA Life Sciences Brazil
Laboratories and clinical services supporting R&D and quality-control studies for the pharmaceutical, veterinary, cosmetics, and medical-device industries.
Brazilian companies under the QIMA Life Sciences umbrella:
- 20+ years’ experience
- 450 FTEs in 5 locations
- 7000+ m² of Labs