WHAT YOU WILL LEARN IN THIS ARTICLE:
- About Wound Healing
- Why Are Chronic Wounds Different from Acute Wounds?
- Chronic Wounds: Types, Common Features, and Contributing Factors
- Chronic Wounds Pose a Major Challenge in Healthcare
- Managing Chronic Wounds: Key Approaches
- Advances in Chronic Wound Treatment: Current Therapies and Ongoing Research
- Emerging Therapy Solutions
- Market Trends: The Growing Demand for Chronic Wound Care
- Conclusion and Outlook
Discover related blog articles
Skin wounds represent a substantial clinical and economic burden worldwide, affecting both patients and healthcare systems. They are defined as injuries or pathological alterations compromising skin integrity, arising from a wide range of causes, including extrinsic factors (e.g., pressure, burns, and cuts), surgery, infection, and autoimmune or pathologic conditions such as diabetes. Wounds are conventionally classified as either acute (healing) or chronic (non-healing) depending on their underlying causes and consequences.
It is estimated that nearly one billion individuals globally are affected by acute or chronic wounds, reflecting a growing global concern that extends far beyond individual discomfort.
In the context of increasing comorbidities such as diabetes, cardiovascular disease, and population aging, distinguishing between acute and chronic wounds has become increasingly important for guiding targeted and effective care. A thorough understanding of their underlying differences is crucial for the development of appropriate prevention and treatment strategies.
Chronic wounds, in particular, present multifactorial challenges that hinder healing. Addressing these complexities is critical to improving clinical outcomes – namely, achieving faster and more sustained wound closure – resulting in improved patient quality of life and reduced healthcare costs.
About Wound Healing
Wound healing is a complex and dynamic process designed to efficiently restore damaged tissue. It is divided into four interconnected and overlapping phases (Figure 1):
- Hemostasis: Immediately after vascular injury, the damaged vessel undergoes rapid vessel contraction to limit blood loss at the site of the injury. Platelets adhere to the damaged vessel wall, activate, and aggregate to form the platelet plug. This plug is eventually stabilized by a dense fibrin mesh formed through the coagulation cascade. In addition, the injury – particularly through the activation of platelets – can trigger the release of various growth factors and chemotactic factors as well as damage-associated molecular patterns (DAMPs) providing a foundation for subsequent events in the wound healing process.
- Inflammation: The inflammatory phase follows, aiming to remove debris and pathogens and to prevent infection through the activation of immune cells. This activation is often triggered by microbial components entering the skin through the compromised barrier or DAMPs. The process begins with the rapid recruitment of neutrophils to the wound site, driven by histamine release from mast cells. Recruited from damaged vessels by chemoattractants, neutrophils initiate the initial inflammatory response. As the process progresses, monocytes are subsequently recruited, differentiating into macrophages that contribute to the clearance of remaining cell debris and neutrophils. Moreover, immune cells release growth factors and cytokines that recruit key players including stem and progenitor cells, fibroblasts, keratinocytes, and endothelial cells, to initiate the proliferation phase.
- Proliferation: During the proliferation phase, granulation tissue forms and tissue regeneration is initiated. Fibroblasts migrate to the wound site, replacing the initial fibrin clot with a more substantial granulation tissue, essential for dermal reconstruction. In parallel, endothelial cells drive angiogenesis, ensuring the establishment of a robust vascular network to support the growing tissue. Meanwhile, keratinocytes located at the wound edges proliferate and migrate to reconstitute the epidermal barrier and close the wound through re-epithelialization.
- Remodeling (Maturation): In the final phase, collagen fibers undergo maturation, becoming more organized and cross-linked. Excess cells undergo apoptosis, while extracellular matrix (ECM) proteins are deposited and degraded in a balanced manner to restore proper tissue architecture and function.
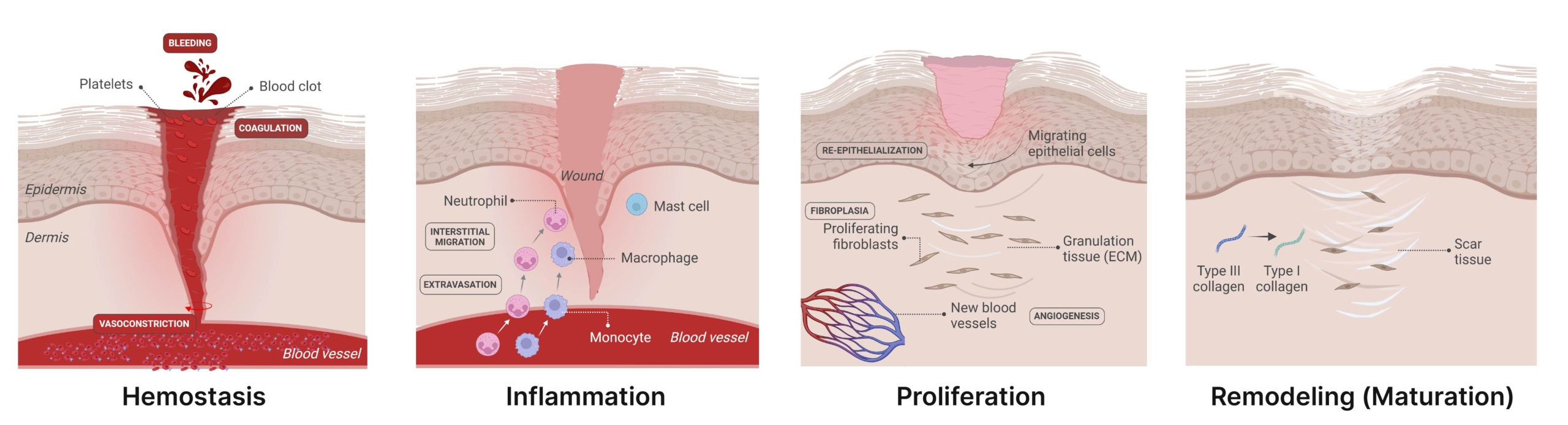
Figure 1. The stages of wound repair and their major cellular components. Wound repair begins with hemostasis, where the formation of a platelet plug limits blood loss, and a provisional fibrin matrix is formed. This is followed by the inflammatory phase, marked by the recruitment of immune cells – including neutrophils, macrophages, monocytes, and mast cells – to the wound site in response to damage signaling factors. These cells play a critical role in preventing infection and clearing remaining cell debris. During the proliferative phase, keratinocytes migrate to close the wound gap, endothelial cells drive angiogenesis to restore the vascular network, and fibroblasts replace the initial fibrin clot with granulation tissue. Macrophages and regulatory T cells (Tregs) are also crucial for this stage of healing. Finally, the deposited matrix is remodeled further by fibroblasts, blood vessels regress and myofibroblasts induce overall wound contraction (From Wilkinson and Hardman, 2020).
Why Are Chronic Wounds Different from Acute Wounds?
Acute wounds encompass a variety of skin injuries resulting from diverse causes, including surgical incisions, trauma (burns, lacerations, abrasions), exposure to radiation, extreme temperature changes, or chemical agents. Clinically, uncomplicated acute wounds are marked by erythema, swelling, warmth, and purulent discharge if infection occurs. The healing of acute wounds generally occurs within 4 to 12 weeks, depending on factors such as the nature, severity, size and depth of the injury as well as the patient’s age, comorbidities and post-injury care. This process results in the restoration of functional and anatomical integrity of the skin through an organized traditional wound healing process. In acute wounds, the four phases occur seamlessly and predictably, resulting in timely closure and tissue restoration.
Unlike acute wounds, chronic wounds fail to progress through the normal phases of healing and remain trapped in a dysregulated inflammatory state. Chronic wounds are difficult to heal due to previous pathological conditions such as diabetes, autoimmune disorders, venous stasis, infection, inflammation, tumors, or exposure to physical agents. Unlike acute wounds, chronic wounds exhibit a significantly delayed healing process (typically remaining unhealed after 12 weeks). These wounds often drain, emit odors, and are associated with pain and tenderness. Without the protection of an intact skin envelope, these wounds can be retraumatized and progress in size.
Most chronic wounds are characterized by a prolonged or excessive inflammatory phase, marked by an overabundant infiltration of neutrophils, persistent infections, frequent formation of atypical tissue and organ biofilms, and the inability of dermal or epidermal cells to respond to reparative stimuli. This prolonged inflammatory state often fails to progress to the next phases of wound healing and promotes excessive recruitment of immune cells and the release of growth factors to the wound site.
Due to prolonged activation of pro-inflammatory cytokine cascades that are otherwise tightly regulated during acute wound healing, chronic wounds display elevated levels of proteases such as matrix metalloproteinases (MMPs). Among them, MMP-2 and MMP-9 degrade the ECM and prevent the initiation of the proliferative stage of healing. Additional hallmarks include reduced mitogenic activity, overproduction of reactive oxygen species (ROS), reduced concentrations over time of growth factors, and decreased levels of tissue inhibitors of metalloproteinases (TIMPs) compared to acute wounds, and impaired angiogenesis. In addition, chronic wounds are also characterized by:
- accumulation of senescent cells, with impaired proliferative capacity and altered secretory phenotype, preventing cells to respond to classical wound healing signals;
- a deficiency of stem cells that are often also dysfunctional;
- critical bacterial colonization and the frequent formation of drug-resistant microbial biofilms.
Collectively, these cellular and molecular alterations are key contributors to impaired tissue regeneration and the establishment of wound chronicity.
Table 1. Comparative characteristics of acute and chronic wounds (modified from Morton and Philipps, 2016) [21]. MRSA, Methicillin-resistant Staphylococcus aureus.
| Characteristics | Acute wounds | Chronic wounds |
|---|---|---|
| Wound healing time | 4-12 weeks | More than 12 weeks |
| Bacteria | Low levels of bacteria | High levels of bacteria (MRSA) |
| Inflammation | Transient and controlled | Prolonged and excessive |
| Macrophage polarization | M1-like phenotype → M2-like phenotype transition (normal resolution of inflammation) | Impaired M1-like phenotype → M2-like phenotype transition (persistent inflammation) |
| Neutrophil infiltration | Early, transient, and resolved | Excessive, sustaining inflammation |
| Inflammatory cytokines | Low inflammatory cytokine levels | High inflammatory cytokine levels |
| Proteases | Regulated proteases | High protease levels |
| ROS production | Moderate/regulated ROS | High ROS levels |
| Oxygenation | Adequate tissue oxygenation | Hypoxia (reduced oxygenation) |
| Cellular phenotype | Normal cell phenotype | Altered cellular phenotype |
| Matrix | Intact functional matrix | Degraded nonfunctional matrix |
| Mitogenic activity | High mitogenic activity | Low mitogenic activity |
| Angiogenesis | Induced and effective | Impaired or insufficient |
| Stem cells | Activated, proliferate, migrate, and differentiate | Dysfunctional, exhausted, or unresponsive |
| Senescent cells | Absent or minimal (presence of mitotically competent cells) | Accumulated |
| Cell recruitment | Balanced | Dysregulated |
| Granulation tissue formation | Normal and transient | Defective |
| Epithelial cell differentiation | Proper keratinocyte migration, proliferation, and differentiation leading to effective re-epithelialization | Impaired keratinocyte migration and differentiation |
| Epigenetic regulation | Normal epigenetic activation of wound healing genes | Dysregulated epigenetic mechanisms |
| DNA repair | Functional DNA repair systems handle transient oxidative stress effectively | Impaired DNA repair due to chronic oxidative stress |
Chronic Wounds: Types, Common Features, and Contributing Factors
According to the Wound Healing Society, chronic wounds are classified into four main categories based on their underlying causes, each presenting distinct characteristics in terms of typical location, depth, and appearance: pressure ulcers, diabetic ulcers, venous ulcers and arterial insufficiency ulcers. Despite their different etiologies, these wounds share several common features (e.g., prolonged or excessive inflammation, persistent infections). These pathophysiological mechanisms lead to the failure of these wounds to heal.
For example, chronic wounds such as diabetic ulcers exhibit delayed wound healing due to a combination of systemic and local contributing factors. Systemic factors include aging, sex hormones, stress, malnutrition, obesity, habits (like alcoholism and smoking), chronic medical conditions (including diabetes, endocrine and vascular disorders), medications such as corticosteroids and immunosuppressants, immunocompromised conditions including cancer, radiation therapy, AIDS. Local factors comprise pressure, infection, edema, and/or arterial and venous insufficiency.
Chronic Wounds Pose a Major Challenge in Healthcare
Chronic wounds represent a growing challenge for modern healthcare and constitute a major public health concern due to their significant impact on patients’ quality of life. Their increasing prevalence is largely fueled by population aging and the rising incidence of diabetes and obesity.
Beyond impaired tissue repair, chronic wounds are linked to loss of function and mobility, psychological distress – including depression, anxiety, embarrassment, and social isolation. Moreover, nonhealing wounds, left untreated and poorly managed, may progress to significant medical complications, including infection, sepsis, the need for limb amputation, and even death.
Chronic wounds predominantly affect the elderly population. In the United States, 2% of the total population suffers from chronic wounds, with similar data reported in European countries.
In addition to the personal and familial toll, chronic non-healing wounds contribute to prolonged hospitalizations and generate substantial healthcare costs worldwide encompassing general practitioner (GPs) visits, community nurse visits, outpatient attendances, hospital admissions and day cases, drug prescriptions and wound care products (e.g., dressings). In developed countries, the management of chronic wounds accounts for an estimated 1-3% of total healthcare expenditures. In the United States, the overall costs related to chronic wounds are approximated to be 50 billion USD per year.
Managing Chronic Wounds: Key Approaches
The effective care of chronic wounds requires a multimodal approach, that includes wound bed optimization, management of underlying chronic conditions, and regular follow-up. The accurate diagnosis of wounds should follow an orderly fashion, ruling in or out systemic (e.g., diabetes mellitus, malnutrition) and regional (i.e., neuropathic, arterial, venous, or lymphatic or combinations of these) etiologies first. Once these factors have been evaluated, attention should then shift to local factors – such as unremitting pressure, trauma, or non-healing burns.
Advanced techniques such as polymerase chain reaction (PCR) for bioburden assessment are becoming more readily available and may contribute to accuracy of diagnosis.
In clinical settings, the cornerstone of chronic wound treatment is the TIME principle, which summarizes the main factors that negatively interfere with the healing process:
- Tissue debridement (in all cases except arterial ulcers)
- Infection/Inflammation
- Moisture imbalance
- Epithelial edge advancement
This framework provides a systematic method for assessing and managing most chronic wounds as well as preparing the wound bed to promote healing.
After addressing these general measures, treatment should be specific to the ulcer type and may comprise exercise, offloading, microclimate, nutritional support, or compression. Interestingly, a panel of experts unanimously recognized that wounds and wound-related procedures are frequently associated with pain, and managing the pain is critical to the patient’s experience.
The ultimate goal of wound care is to achieve complete and timely wound closure, although this is not always achievable. Chronic wounds are particularly challenging to manage effectively. Most clinical guidelines are still evolving, driven by continuous improvements in wound pathology, healing, and therapeutic agents.
Advances in Chronic Wound Treatment: Current Therapies and Ongoing Research
Therapies are designed to debride the wound bed, correctly balance the moisture, control infection, and inflammation, and promote proper re-epithelialization while minimizing excessive contraction.
Patient outcomes might encompass wound-specific clinical measures – such as healing time and wound size reduction – as well as improvement in quality of life. For patients with a realistic prospect of healing, an aggressive approach to achieving that outcome is recommended. Conversely, when healing is no longer a realistic goal, as in palliative wound care, the focus shifts toward pain reduction, exudate management, odor management, and/or other quality-of-life benefits to wound care.
Systemic administration
Systemic antibiotics, whether administered parenterally or orally, have been employed to improve symptom management and reduce further deterioration and infections, in particular clinically invasive infection. Nevertheless, their capacity to penetrate wound biofilms is limited. Another systemic strategy is the administration of antibodies (e.g., infliximab – anti-TNF-α – in the treatment of recalcitrant ulcerating necrobiosis lipoidica) or peptides (e.g., neuropeptide α-melanocyte-stimulating hormone – α-MSH). In addition, certain systemic drugs (e.g., aspirin, flavonoids, sulodexide) may help enhance the wound environment; however, their use requires careful consideration to ensure the potential benefits overcome their associated risks.
Despite beneficial effects in certain cases, systemic administration remains limited due to the difficulties with tissue targeting and the off-target adverse effects. Consequently, for wound management, the direct delivery of bioactive compounds to the specific wound site is generally preferred, being increasingly accepted as the most appropriate delivery strategy for chronic wounds.
Local therapies
Physical treatments
Debridement remains a traditional and central aspect of wound management. Regular maintenance debridement – surgical, biosurgical, mechanical, autolytic, chemical or enzymatic – has been shown to stimulate the wound healing process. Several other physical therapies have demonstrated satisfactory outcomes in the treatment of chronic wounds, including compression therapy, hyperbaric oxygen therapy (HBOT), electrical stimulation, NPWT, or even photobiomodulation and ultrasounds.
Pharmacological treatments
Drugs as topical antibiotics, that reduce bacterial and fungal bioburden in chronic wounds, or antiseptics (e.g., iodine, chlorhexidine) have been widely used topically in wound care and have demonstrated clear clinical benefits. In addition, ointments containing collagenase and dexpanthenol have been employed for improving the healing process as they control the proliferation of fibroblasts, and accelerate re-epithelialization and ECM remodeling. In recent years, topical formulations incorporating growth factors have been investigated in wound healing due to their beneficial effects. However, despite the absence of significant side effects, the limited bioavailability of growth factors, resulting from their rapid clearance from the wound site, restricts their use. In order to alleviate this shortcoming, innovative approaches involving nanoparticle-encapsulated growth factors with improved stability and bioavailability have been topically applied to the wound area.
Skin grafting
Skin grafting is a surgical procedure used to reconstruct skin defects across various anatomical regions, without bringing its own blood supply with it. It is considered the gold standard for the management of thermal injuries. Depending on the source, skin grafts may be classified as autografts (from the same patient), allografts (from another dead or alive patient), or xenografts (from other animals/species). However, these different grafts present several drawbacks, including pain, limited and scarce donor sites, high cost and availability issues, immune rejection, and the risk of disease transmission.
Dressings
A large number of dressing therapies have also been developed to meet multiple objectives in wound care. These include maintaining a moist environment while ensuring high oxygen permeability, protecting the wound bed from physical and mechanical stress, providing a temporary matrix for cell migration, ECM deposition, and neovascularization, as well as reducing pain, providing compression or offloading and releasing drugs (e.g., antibiotics) and bioactive molecules. The materials used in these biomaterial-based systems are diverse, ranging from biological sources – such as fibrin, fibronectin, gelatin, collagen, alginate, chitosan, and hyaluronan – to synthetic compounds like polyglycolic acid (PGA), polylactic acid (PLA) or poly-acrylic acid (PAA). Dressing therapies may be classified into films, non-cellular scaffold-based therapies, bioengineered living skin equivalents – mono/bi-layered cellular therapy mimicking the skin structure – and stem cell-loaded scaffolds. Each type of therapy offers a specific balance between ease of use, bioactivity, and cost, with indications varying according to the wound type (superficial, chronic, deep, or complex).
Emerging therapy solutions
Although these therapeutic modalities are well-studied and can be easy to employ, they still present several limitations. In addition, the wound healing process involves numerous complex cellular and molecular mechanisms, whose impairments remain poorly understood, meaning that no single treatment is sufficient to ensure complete healing.
Consequently, a wide range of emerging and innovative treatment strategies have been investigated in recent years. Their common objective is to promote high-quality wound healing in chronic wounds by providing precise and spatiotemporally controlled treatments that accelerate healing, while meeting patient needs in terms of safety, effectiveness, cost-efficiency, and cosmetic outcomes. Promising alternatives include bioengineered skin substitutes, vacuum-assisted wound closure, stem cell therapy, or growth factor/cytokine therapy. However, most of these approaches still lack assessment and effectiveness in large scale studies. The recent advances in biotechnology, material science, and precision medicine have further expanded the therapeutic arsenal with cutting-edge strategies – ranging from preclinical concepts to clinically available therapies – such as platelet-rich plasma (PRP)-based treatment, ECM-based strategies, cold atmospheric plasma (CAP) therapy-based approaches, extracellular vesicles, nanotherapeutics, microRNA (miR)-based strategies, 3D bioprinting and CRISPR/Cas9 technology. Beyond treatment, the ultimate objective of wound management is to enhance diagnostic and prognostic tools, enabling the design of personalized therapeutic strategies. In this regard, multiomics – an integrated approach combining genomics, transcriptomics, proteomics, and metabolomics – and artificial intelligence (AI) approaches hold great promise for advancing personalized medicine in wound healing. Furthermore, future strategies may adopt combinational approaches that target multiple pathways within a single phase of wound healing or span across different healing stages. These interventions could be applied simultaneously, sequentially, or dynamically adapted in response to the wound’s progression. Accordingly, future research directions are increasingly moving toward more personalized, integrative, and technology-driven approaches.
Market Trends: The Growing Demand for Chronic Wound Care
The global chronic wound care market was valued at USD 12.8 billion in 2022 and is expected to grow at a CAGR of 4.1% between 2023 and 2030. This expansion is mainly fueled by the rising incidence of sports-related injuries, the aging population, the increasing incidence of chronic diseases such as diabetes, cancer, and autoimmune disorders, as well as lifestyle changes including obesity. In addition, as chronic diseases become more widespread, the incidence rate of chronic wounds is anticipated to increase accordingly on a global scale.
The rising demand for effective wound care is further supported by creating general awareness of earlier detection and treatment.
In response, the market is witnessing a growing adoption of advanced wound dressings and related medical devices, with continuous innovation driving the development of novel promising therapeutic options.
Advanced wound dressings dominated the product segment in 2023 and are projected to exhibit the fastest growth rate over the forecast period. Hospitals remained the leading end-users in 2023. Even diabetic foot ulcers represented the largest application segment in 2022 (i.e., 35% of the market), pressure ulcers are anticipated to register the fastest growth in the coming years.
Geographically, North America generated the highest revenue in 2022 – accounting for over 45% of the market, but the Asia-Pacific region is expected to experience the most significant growth through 2030.
Table 2 summarizes the segmentation of the global chronic wound care market based on data from Grand View Research, The Brainy Insights, and Fortune Business Insights.
Table 2. Market segmentation of global chronic wound care.
MEA, The Middle East and Africa; UAE, United Arab Emirates; UK, United Kingdom.
| Product type | Advanced wound dressing: foam dressing, alginate dressing, film dressing, hydrocolloid dressing, collagen dressing, other dressing; Surgical wound care: suture & staples, tissue adhesive & sealants, anti-infective dressing; Traditional wound care: medical tapes, cleansing agents, others; Wound therapy devices: NPWT, oxygen and hyperbaric oxygen equipment, electric stimulation devices, pressure relief devices, others |
| Wound type | Diabetic foot ulcers, pressure ulcers, venous leg ulcers, others |
| End-user | Hospitals, specialty clinics/wound clinics, home care settings, others |
| Regional segmentation | North America, Europe including Germany, France, UK, Italy and Spain, Asia Pacific (e.g., Japan, China, India) and MEA (e.g., South Africa, Saudi Arabia, UAE) |
Conclusion and Outlook
Despite considerable therapeutic advances, chronic wound treatment remains challenging mainly due to complex biological mechanisms and the need for intensive long-term care.
Conventional approaches, including growth factor delivery, wound dressings, and skin grafts, are routinely employed to promote wound healing. However, these treatments often fall short, particularly for chronic or complex wounds.
To overcome these limitations and improve our understanding of wound healing, recent years have seen the emergence of innovative strategies – including stem cell-based therapies, ECM-based strategies, nanotherapeutics, and multiomics approaches – aiming to promote safe, efficient, and tailored healing of chronic wounds.
While these promising developments open new avenues and bring renewed hope for patients suffering from chronic wounds, translating them into clinically applicable therapies remains a key challenge. This requires optimizing cost-effectiveness, ensuring compliance with strict regulatory pathways, and meeting critical criteria such as safety, reproducibility, and efficacy.
Looking ahead, the integration of an enhanced understanding of chronic wound biology with technological innovation holds significant promise for improving therapeutic outcomes. In particular, the integration of advanced therapeutic approaches – including emerging technologies, combinational approaches, and personalized medicine – are expected to shape the next generation of wound care.
To achieve this goal, collaborations between academia, industry, clinicians, government bodies, and contract research organizations (CROs) will be essential, not only to advance the understanding of chronic wound pathophysiology but also to translate scientific insights into effective and clinically applicable therapies.
In this context, CROs serve as essential partners throughout the entire process of wound care product development. Through their expertise in preclinical studies, clinical trial management, and regulatory affairs, CROs help pharmaceutical and biotechnology companies accelerate the discovery and development of novel therapies. Their contribution helps optimize resource allocation, reduce development timelines, and enhance the overall quality and robustness of R&D programs.
Abbreviations
AIDS, Acquired immunodeficiency syndrome; CAGR, Compound annual growth rate; CAP, cold atmospheric plasma; CRISPR, Clustered regularly interspaced short palindromic repeats; CRO, Contract research organizations; DAMPs, Damage-associated molecular patterns; ECM, Extracellular matrix; EMT, Electromagnetic therapy; HBOT, Hyperbaric oxygen therapy; MMPs, Matrix metalloproteinases; NPWT, Negative pressure wound therapy; PCR, Polymerase chain reaction; PRP, Platelet-rich plasma; ROS, reactive oxygen species; TIMPs, tissue inhibitors of metalloproteinases; TNF-α, Tumor necrosis factor-α.
Written by:
Philippe Gentine, PhD
Life Sciences R&D Expert
Edited by:
Mara Carloni, PhD
Scientific Communications & Marketing Project Leader
References
- Abazari M et al. Int J Low Extrem Wounds. 2022.
- Avishai E et al. EPMA J. 2017.
- Basoulis D et al. Metabolism. 2016.
- Bowers S, Franco E. Am Fam Physician. 2020.
- Choudhary V et al. Int J Mol Sci. 2024.
- Cramer MC, Badylak SF. Ann Biomed Eng. 2020.
- Dabas M et al. Adv Wound Care (New Rochelle). 2023.
- de Souza KS et al. Exp Dermatol. 2015.
- Demidova-Rice TN et al. Adv Skin Wound Care. 2012.
- Ding X et al. Burns Trauma. 2022.
- Dionyssiou D et al. Int Wound J. 2013.
- Djavid GE et al. J Wound Care. 2020.
- Eriksson E et al. Wound Repair Regen. 2022.
- Falcone M et al. JGAR. 2021.
- Freedman BR et al. Sci Adv. 2023.
- Frykberg RG, Banks J. Adv Wound Care (New Rochelle). 2015.
- Garraud O et al. BMC Immunol. 2017.
- Grand View Research. Chronic Wound Care Market Size, Share & Growth Report 2030
- Guest JF et al. J Wound Care. 2017.
- Guo S, Dipietro LA. J Dent Res. 2010.
- Järbrink K et al. Syst Rev. 2016.
- Järbrink K et al. Syst Rev. 2017.
- Jiang Y et al. Int J Mol Med. 2021.
- Jiang Y et al. Front Immunol. 2022.
- Jones RE et al. JAMA. 2018.
- Kolimi P et al. Cells. 2022.
- Krzyszczyk P et al. Front Physiol. 2018.
- Las Heras K et al. J Control Release. 2020.
- Leaper DJ et al. Int Wound J. 2012.
- Mofazzal Jahromi MA et al. Adv Drug Deliv Rev. 2018.
- Morton LM, Phillips TJ. J Am Acad Dermatol. 2016.
- Muller M et al. Diabet Med. 2008.
- Mullin JA et al. Bioeng Transl Med. 2023.
- Munro G. Wound Pract Res. 2017.
- Nicks BA et al. Int J Emerg Med. 2010.
- Olsson M et al. Wound Repair Regen. 2019.
- Ousey K et al. Br J Community Nurs. 2016.
- Phillips CJ et al. Int Wound J. 2016.
- Pinto AM et al. Viruses. 2020.
- Popescu V et al. Biomedicines. 2023.
- Rajendran NK et al. J Drug Deliv Sci Technol. 2018.
- Raissi-Dehkordi N et al. Front Med (Lausanne). 2025.
- Rao KM et al. Int J Biol Macromol. 2025.
- Raziyeva K et al. Biomolecules. 2021.
- Rippon MG et al. J Wound Care. 2024.
- Ross K. J Cell Physiol. 2021.
- Schilrreff P, Alexiev U. Int J Mol Sci. 2022.
- Scridon A. Int J Mol Sci. 2022.
- Sen CK. Adv Wound Care (New Rochelle). 2021.
- Shi C et al. Front Bioeng Biotechnol. 2020.
- Shi S et al. Front Pharmacol. 2025.
- Suthar M et al. J Biomed Sci. 2017.
- Tabriz AG, Douroumis D. J Drug Deliv Sci Technol. 2022.
- The Brainy Insights.
- The Wound Healing Society.
- Tottoli EM et al. Pharmaceutics. 2020.
- Wei X et al. Burns Trauma. 2022.
- Wilkinson HN, Hardman MJ. Open Biol. 2020.
- Zhao R et al. Int J Mol Sci. 2016.