Hair removal is widespread, yet the biological effects of mechanical epilation remain underexplored. This gap was addressed in a collaborative study with the University of Münster and the University of Miami Miller School of Medicine, published by our team in the International Journal of Cosmetic Science (IJCS) (Bertolini et al., 2024) [1]. The publication, which recently received the Best Paper Award from the IJCS at the 2025 Society of Cosmetic Scientists (SCS) Annual Conference, explored the biological responses triggered by mechanical epilation in organ-cultured human scalp skin.
This article provides an overview of the study and explains how its findings offer key insights for cosmetic and dermocosmetic innovation, informing both device design and targeted post-care strategies.
Share This Research Highlight
Hair Removal: Depilation vs. Epilation Strategies
Removing unwanted hair is a common part of personal care for both women and men. Among the most popular mechanical methods are depilation — the removal of the hair shaft from the skin’s surface — and epilation — the removal of the hair shaft from the hair follicle (HF). Epilation methods include tweezing, waxing, sugaring, threading, electrolysis, light-based hair removal, and electro-mechanical devices known as epilators [2,3]. While valued for long-lasting smoothness, epilation can cause pain, irritation, or follicular changes (e.g., folliculitis, hyperpigmentation) [19].
Designed to overcome some of the negative aspects of epilation without compromising complete hair shaft removal [3,4], electro-mechanical epilators typically consist of a rotating barrel with multiple tweezers that remove hairs at high speed (up to 2 m/s), each one grasping a hair and plucking it against the direction of hair growth. Ex vivo investigations on porcine skin have shown that electric epilation and other hair removal methods can promote skin permeation and may compromise epidermal barrier function [5].
Biological Responses to Mechanical Epilation: A New Perspective
While superficial effects of epilation are well-characterized, its biological impact — in particular that of mechanical methods — on the hair follicle and surrounding perifollicular compartments remains far less understood. Given their widespread use, especially in home-based hair removal routines, understanding how these devices affect this complex microenvironment — involved, among other functions, in pigmentation, sebum regulation, neurogenic inflammation, immune homeostasis and broader cellular responses — is particularly relevant.
Over time, repeated mechanical stimulation may gradually disrupt skin homeostasis, affecting pigmentation, barrier integrity, or dermal structure.
Bertolini et al (2024) [1] help address this gap. Using an ex vivo human scalp model, they explored biological responses to electro-mechanical epilation. Beyond advancing scientific knowledge, their results open new perspectives for improving device tolerability and post-epilation skin recovery strategies.
Revealing the Biological Effects of Mechanical Hair Removal: Ex Vivo Study Design
Full-thickness scalp skin was collected from healthy Caucasian women undergoing face-lifting surgery (Figure 1-1). Epilation was standardized using a Braun Silk-épil 7 at >20° angle with low pressure (Figure 1-2).
Controls were either shaved or left untreated. Biopsies were prepared from the epilated or control skin samples (Figure 1-3) and cultured ex vivo (Figure 1-4) according to our previously described organ culture protocol [6,7,8], for up to 6 days.
Samples were snap frozen for analysis at day 0 (40–60 min after treatment; peak inflammation), 3 and 6 (Figure 1-5) and cryosectioned.
Key endpoints included:
- Hair removal and regrowth: Biopsies were photographed at day 0, 3, and 6 before freezing
- Histology and histochemistry: Periodic acid–Schiff (PAS) (to visualize follicular structures and hair shaft removal), Fontana-Masson (to determine melanin content of the HFs), Oil Red O (to assess lipid content of sebaceous glands), toluidine blue (to identify mast cells)
- Immunohistochemistry and immunofluorescence microscopy:
- TUNEL assay (keratinocyte and sebocyte apoptosis);
- Expression of neurogenic skin inflammation, itch, and/or thermosensation (TRPV1, TRPA1, NGF, NKR1);
- Analysis of immunological markers (MHC I/II, CD200, ICAM-1) and bulge stem cell marker (K15).
- Quantitative (immuno-)histomorphometry:
- Melanin, sebum, and marker expression (MHC I, CD200, ICAM-1, NGF, NKR1, TRPV1, TRPA1) were quantified in defined follicular compartments.
- The percentage of apoptotic outer root sheath (ORS) keratinocytes (TUNEL+/DAPI+) was quantified in the infundibulum, bulge, proximal ORS; in the bulb, apoptotic hair matrix keratinocytes were quantified.
- K15+ ORS keratinocytes among DAPI+ were assessed in the bulge, while MHC class II+ cells and mast cells were counted in the perifollicular dermis within 100 μm around the HF.
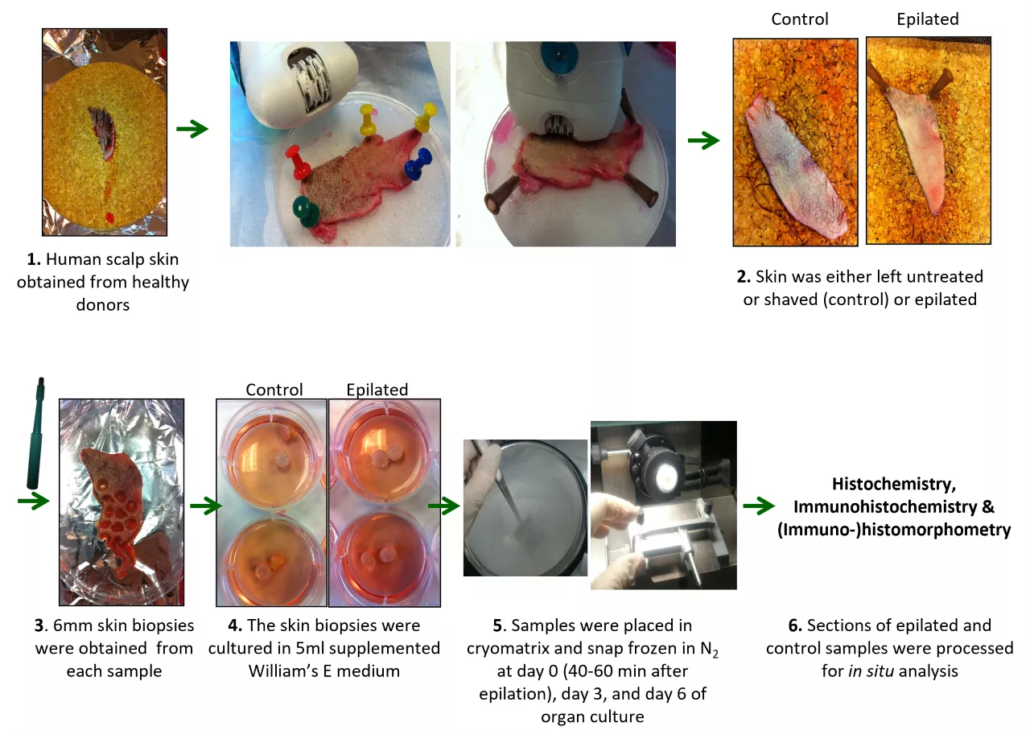
Figure 1: Workflow of human scalp sample analysis and organ culture ex vivo (Bertolini et al, IJCS 2024).
Key Findings: What Happens After Electro-Mechanical Epilation?
Structural Effects
Electro-mechanical epilation ex vivo removed nearly all of the hair shaft while preserving most of the anagen hair matrix and the entire inductive HF mesenchymal structures.
It also resulted in the partial extraction of epithelial HF cells beyond the hair shaft, and induced early alterations of the basal membrane (BM) – thinning or thickening -, which persisted up to six days post-procedure.
This indicates that epilation results in both immediate and enduring damage to the BM, which is likely to impact the function of the HF epithelium over the long term
Melanin Production
Since previous studies have reported hair shaft lightening after repeated plucking, hair follicle melanogenesis was examined.
Three days after epilation, melanin production was significantly reduced in anagen VI HF (Figure 2a), and this decrease persisted for up to six days (Figure 2a,b).
Strikingly, the melanin content was decreased not only in HFs where HF pigmentary unit melanocytes had been extracted alongside the hair shaft but also in those HFs that had retained their pigmentary unit (Figure 2b).
This suggests that epilation-derived HF micro-trauma can inhibit melanogenesis within the HF pigmentary unit even when it is not directly damaged.
Cell Apoptosis
Apoptosis of HF keratinocytes was evaluated, as this has previously been observed following waxing-based epilation or plucking in mouse skin.
Epilation induced marked apoptosis of keratinocytes in the hair matrix and significantly in bulge epithelium as early as 40-60 min post-treatment, with effects persisting up to 6 days.
In contrast, the proximal ORS and the infundibulum were spared. Therefore, while bulge and hair matrix cells appear to be quite sensitive to epilation-induced apoptosis, those in the proximal ORS and infundibulum appear to be resilient.
Moreover, the percentage of K15+ cells in the bulge was not reduced after 6 days. This suggests that the pool of bulge-resident K15+ epithelial HF stem cells is not compromised by electro-mechanical epilation ex vivo, despite increased apoptosis in bulge epithelium.
Preliminary data (from a single donor) also showed increased sebocyte apoptosis in the peripheral zone of sebaceous glands at day 6, while sebum content remained unchanged, and no difference in apoptosis was observed in the maturation zone.
Preliminary data suggest that epilation also promotes apoptosis in sebaceous glands.
Transient Immune Privilege Disruption
Adressing key elements of HF immune privilege (IP) at day 3 revealed significantly upregulated MHC class Ia expression in both the hair bulb and bulge, while the “no danger” signal CD200 was significantly downregulated in the bulge region.
These changes were not present at day 0 or day 6, where expression levels were comparable to controls.
This suggests that epilation transiently compromises HF IP in both the bulge and bulb, but that HFs are able to restore it over time.
Perifollicular inflammation
Epilation induced an increase in MHC class II+ cells at day 3 and significantly at day 6, as well as a significant rise in mast cells at day 3, both around the infundibulum.
Mast cell degranulation was significantly reduced at days 0 and 6 in this area, but increased significantly at day 6 in the suprabulbar and bulbar area.
In parallel, ICAM-1 expression was unexpectedly and significantly decreased in both bulge and bulb mesenchyme (at 40–60 min), then significantly upregulated at day 3 in the perifollicular connective tissue sheath (CTS) around both regions in epilated HFs. At day 6, ICAM-1 remained upregulated, but only in the bulge.
These findings suggest that epilation induces sustained pro-inflammatory responses in the HF mesenchyme and perifollicular skin, even without blood-derived immune cell influx.
Neurogenic Inflammation and Sensory Markers
Since human skin is denervated under standard organ culture conditions—making sensory measurements impossible—surrogate markers for neurogenic inflammation, thermoregulation, itch, and/or pain were assessed.
NGF expression was initially downregulated, then significantly upregulated at day 3 in the upper ORS, and normalized by day 6.
NK1R was significantly upregulated at days 0 and 3 in several HF compartments (isthmus, bulge, hair bulb), then normalized by day 6.
TRPV1 was also significantly upregulated at days 0 and 3 in the isthmus and bulge of the HF, but downregulated at day 6.
Finally, TRPA1 was significantly downregulated only at day 6.
Although based on a single donor, these findings suggest that epilation transiently alters neurogenic inflammation and sensory markers, potentially increasing skin reactivity to irritation-triggering stimuli.
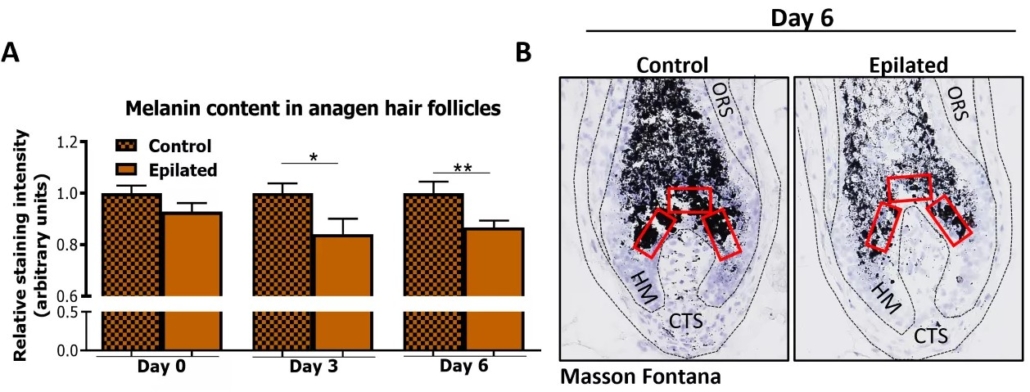
Figure 2: Hair follicle (HF) melanin content is decreased after epilation. (a) Quantitative histomorphometry of melanin content in anagen VI HFs still maintaining pre-cortical hair matrix after epilation and from control samples 40–60 min after epilation (day 0) and at days 3 and 6 post-epilation. The relative staining intensity normalized to the respective control group of each day is shown. N= 7–24 anagen HFs/group from 2–3 independent experiments (3 (day 0, day 3) and 2 (day 6) donors with two punches per experimental group per donor). Mean ± SEM. [ […] *p < 0.05, **p < 0.01. (b) Representative images of Fontana-Masson histochemistry depicting reference areas for evaluation (red squares). CTS, connective tissue sheath; HM, hair matrix; ORS, outer root sheath (Bertolini et al, IJCS 2024).
Implications and Future Directions
This pilot study offers the first evidence that HF micro-trauma caused by mechano-electrical epilation triggers complex biological responses that extend beyond hair shaft removal and affect HF physiology over time.
These responses include persistent focal thinning and thickening of the HF basal membrane, apoptosis in HF keratinocytes and sebocytes, reduced melanin content without reduction of K15+ epithelial HF stem cells, transient disruption of HF IP accompanied by the induction of peri- and intrafollicular pro-inflammatory responses, along with temporary modulation of key neurogenic markers involved in inflammation, itch, and/or thermosensation.
Together, these mechanisms may help explain post-epilation effects commonly reported with repeated use — including delayed hair regrowth, thinner and lighter hair, and transient skin discomfort.
That said, due to limited access to human scalp skin, some experiments relied on a single donor, warranting confirmation in future studies involving additional donors.
Scientific and Technical Perspectives
Our study highlights the value of full-thickness human scalp skin organ culture as a promising ex vivo model to explore the biological impact of epilation. Despite inherent limitations — notably the absence of innervation, blood perfusion, and circulating immune cells — it preserves key follicular and perifollicular structures, enabling standardized, multi-day follow-up of structural and molecular changes.
Looking ahead, several avenues could further enhance its relevance:
- We have recently developed a human skin reinnervation system [9] that may overcome denervation and enable more accurate investigation of pain-related signaling.
- Adapting culture conditions to reduce reliance on antibiotics and disinfectants may preserve the native physiological skin and HF microbiome.
- Methodological improvements are also needed to address the absence of blood perfusion, immune cells, mediators, and metabolites.
- Broadening biomarker panels to include genes and pathways involved in wound-induced HF neogenesis [10], alopecia-associated tissue remodeling [11], or epigenetic memory of cutaneous injury [12] may clarify long-term follicular responses to repeated epilation.
- Integrating transcriptomic approaches across multiple timepoints would refine understanding of dynamic tissue responses.
- Finally, this model could support future studies on neuroendocrine signaling [13,14,15], HF innervation [16], and HF microbiome [17,18], despite current constraints.
These refinements would strengthen its utility for both mechanistic and preclinical studies.
Translational Findings for Cosmetic and Dermocosmetic Applications
Post-Epilation Care
The study underscores the need for targeted post-care formulations designed to modulate the biological cascade triggered by electro-mechanical epilation. Neuro-soothing, anti-inflammatory, immune-balancing, or barrier-supporting agents — delivered through serums, creams, or hydrogel patches — may help limit discomfort and reduce longer-term reactivity.
These pathways and markers may inform efficacy studies and support claim substantiation.
Rethinking Device Design
This study invites a reassessment of how epilation devices interact with the skin. The biological responses observed — including inflammation, tissue remodeling, and sensory pathway activation — suggest that device performance should go beyond efficiency to include biological compatibility.
Future designs may integrate sensors or adaptive features capable of adjusting intensity or motion based on tissue response, thereby better respecting skin physiology.
Such innovations may improve comfort and reduce adverse effects, particularly for sensitive skin or individuals prone to pigmentation — while offering opportunities for brand differentiation.
A synergistic approach combining device design with post-care formulation may further minimize side effects and extend benefits.
New Tools for Preclinical Validation
The ex vivo model developed here could provide a human-relevant platform to evaluate devices and topical products before clinical testing.
It enables the quantification of key responses — including inflammation, neurosensory activation, and pigmentation shifts — that are rarely explored in standard performance assessments.
Aligned with current efforts to reduce animal testing, this model may also support investigations into other epilation techniques (e.g., waxing, laser), microbiome-related effects, and interindividual variability across skin types and phototypes.
It provides a valuable tool to support innovation and ensure skin-compatible hair removal solutions.
Abbreviations:
BM, basal membrane; CD200, Cluster of Differentiation 200; CTS, connective tissue sheath; DAPI, 4′,6-diamidino-2-phenylindole ; HF, hair follicle; HM, hair matrix; ICAM-1, Intercellular adhesion molecule 1; IP, immune privilege; K15, Cytokeratin 15; MHC I/II, Major histocompatibility complex class I/II; NGF, Nerve growth factor; NKR1, Neurokinin-1 receptor; ORS, Outer root sheath; PAS, Periodic acid–Schiff; TRPA1, Transient receptor potential cation channel subfamily A member 1; TRPV1, Transient receptor potential cation channel subfamily V member 1; TUNEL, Terminal deoxynucleotidyl transferase dUTP nick end labeling.
Written by:
Philippe Gentine, PhD
Life Sciences R&D Expert
Edited by:
Sabrina Hoefling, PhD
Innovation Marketing Project Leader
Last update 05/11/2025