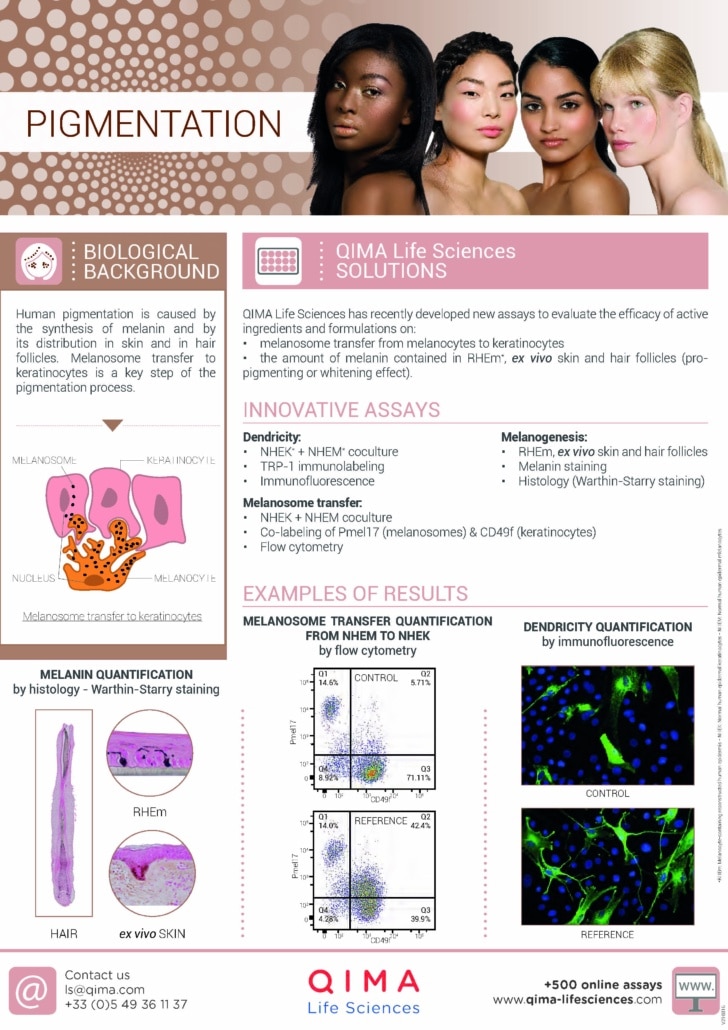
Hyperpigmentation has long been approached through the lens of melanocyte biology alone. Yet, recent advances in dermatological science reveal a more intricate story, one in which pigmentation is the product of dynamic interactions between multiple skin cell types, aging pathways, and inflammatory signals. From the epidermis to the dermis, from cellular senescence to vascular and sebaceous influences, pigmentation emerges as a multicellular, systemic process.
This shift in perspective not only reshapes our understanding of pigmentary disorders like melasma and post-inflammatory hyperpigmentation but also opens new avenues for therapeutic innovation. Targeting the skin’s microenvironment, and not just melanogenesis, is now key to achieving lasting results in a wide range of skin tones.
Discover related blog articles
Personalized skin care to address skin pigmentation diversity
1. Rethinking Pigmentation: Beyond the Melanocyte-Centric Model
1.1 The classical model: Melanocytes and melanin synthesis
Pigmentation of the skin is classically attributed to the activity of melanocytes, specialized dendritic cells located in the basal layer of the epidermis. These cells form the epidermal melanin unit, an interactive structure in which one melanocyte supports approximately 30-40 surrounding keratinocytes through melanosome transfer. Melanin, the pigment responsible for skin, hair, and eye color, is synthesized within melanosomes through a complex enzymatic cascade known as melanogenesis. The two main forms of melanin, eumelanin (brown to black) and pheomelanin (yellow to red), are produced in varying proportions depending on genetic, hormonal, and environmental influences.
This traditional view of pigmentation has long dominated dermatological research and cosmetic development. Hyperpigmentation disorders such as melasma or post-inflammatory hyperpigmentation (PIH) have therefore been addressed primarily by targeting melanocytes or their melanin-producing enzymes, such as tyrosinase.
Interested in our capabilites?
Download our flyer on
Skin Pigmentation
1.2 A multicellular orchestration
Skin pigmentation is not solely dictated by melanocyte activity.
Instead, it results from dynamic cross-talk between various cell types within the epidermis and dermis, all orchestrated by the surrounding microenvironment (See Figure 1).
This interplay not only modulates melanocyte function but can also alter their morphology; in chronic conditions, melanocytes may become pendulous, protruding into the dermis as a result of junctional degradation and local stress. Chronic pigmentary disorders such as melasma exemplify this complex, multicellular regulation.
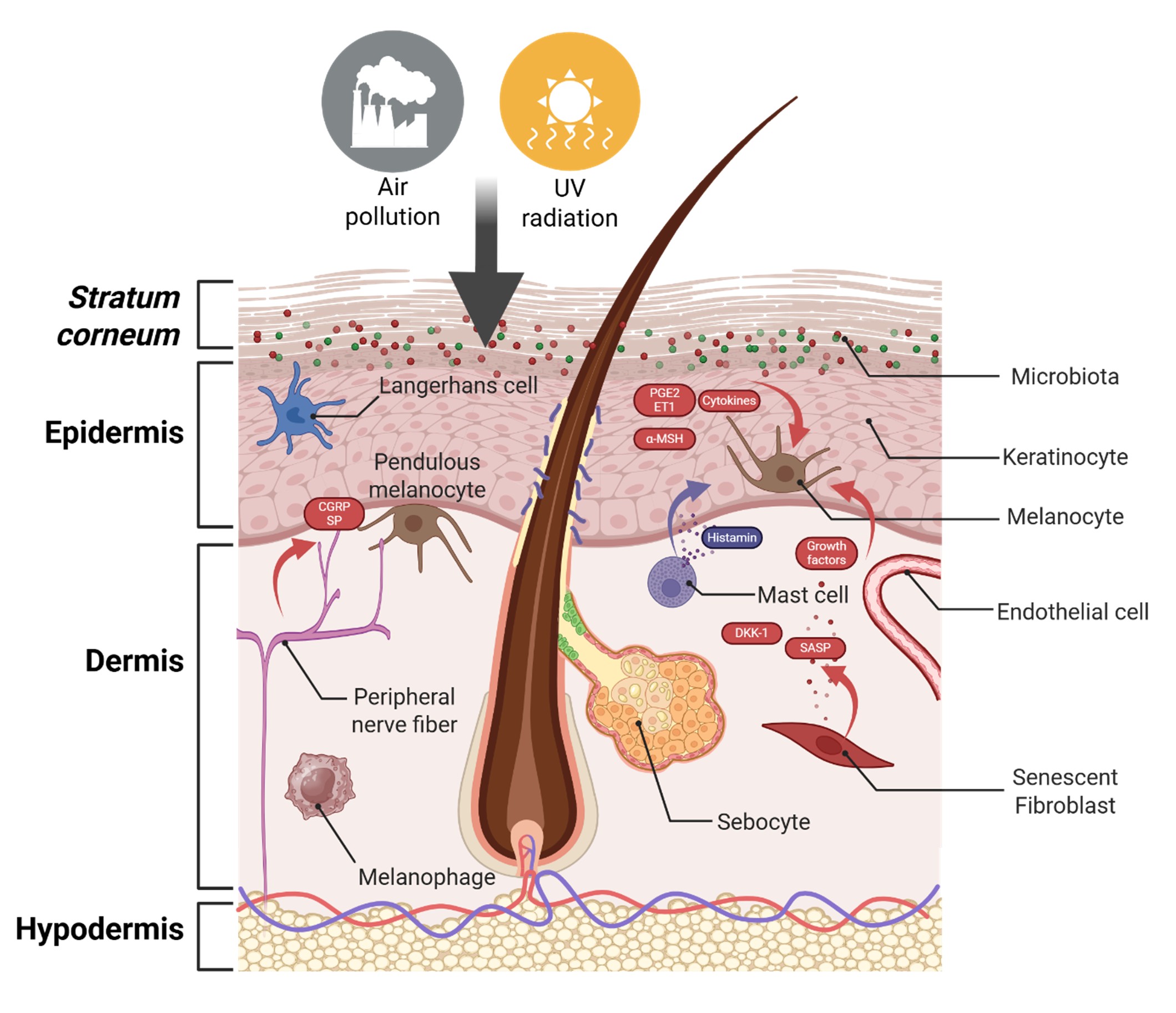
Figure 1. A multicellular orchestration of pigmentation. Environmental stressors such as UV radiation and air pollution disrupt skin homeostasis and activate multiple skin cell types. Through the release of pro-inflammatory mediators (e.g., cytokines, prostaglandins, endothelin-1), neuropeptides (CGRP, SP), and senescence-associated signals (SASP), these cells cooperatively reshape the cutaneous microenvironment, leading to altered melanocyte behavior and persistent pigmentation disorders such as melasma. Figure created with biorender.com.
CGRP: Calcitonin Gene-Related Peptide; ET1: Endothelin-1; PGE2: Prostaglandin E2; SASP: Senescence-Associated Secretory Phenotype; SP: Substance Pα-MSH: Alpha-Melanocyte Stimulating Hormone; DKK1: Dickkopf-1 (Wnt pathway antagonist).
ROLE OF KERATINOCYTES
Keratinocytes, the primary recipients of melanosomes, play a pivotal role in regulating melanocyte behavior. They influence melanogenesis through the secretion of cytokines such as IL-1α and IL-6, prostaglandins like PGE2 and PGF2α, and various oxidative stress mediators. Under environmental stimuli, including UV radiation, pollution, or barrier disruption, these signals can either stimulate or suppress pigment production.
Additionally, keratinocytes produce proopiomelanocortin (POMC), a precursor of α-MSH, which binds to melanocortin-1 receptors (MC1R) on melanocytes, promoting melanin synthesis and melanocyte survival. More recently, extracellular vesicles (EVs) secreted by keratinocytes have been shown to carry bioactive molecules such as miRNAs, proteins, and signalling lipids that modulate melanocyte activity and pigment transfer, adding a new layer of complexity to this intercellular dialogue.
ROLE OF DERMAL FIBROBLASTS
Dermal fibroblasts are emerging as critical regulators of pigmentation, particularly in pathological contexts such as melasma.
They influence melanocyte function via paracrine signalling, secreting key factors such as stem cell factor (SCF), keratinocyte growth factor (KGF), basic fibroblast growth factor (bFGF), and Wnt modulators. These factors can either enhance or suppress melanogenesis depending on the physiological or pathological state of the fibroblasts.
In melasma lesions, fibroblasts often display senescent features, including altered morphology, increased β-galactosidase activity, and a pro-melanogenic secretome enriched in HGF, SCF, and MMPs. This senescence-associated secretory phenotype (SASP) contributes to chronic pigmentation by promoting melanocyte activation and altering the extracellular matrix. Importantly, targeting senescent fibroblasts has been proposed as a promising strategy for reducing persistent hyperpigmentation, especially in photoaged or inflamed skin.
ROLE OF MAST CELLS
Mast cells, traditionally known for their role in allergic and inflammatory responses, are increasingly recognized as modulators of pigmentation, particularly in chronic pigmentary conditions like melasma.
In lesional skin, mast cell density is often significantly elevated. These cells secrete a range of bioactive mediators, including histamine, tryptase, heparin, TNF-α, and IL-17, that can indirectly stimulate melanogenesis by influencing melanocytes, fibroblasts, and vascular cells. Histamine, for example, can upregulate tyrosinase activity in melanocytes via H2 receptors, enhancing melanin production.
Mast cells also contribute to angiogenesis and extracellular matrix remodeling, which are key histopathological features of melasma. Their sustained activation may help sustain a chronic low-grade inflammatory state, creating a microenvironment conducive to pigment overproduction. This positions mast cells as potential therapeutic targets in the management of recalcitrant hyperpigmentation.
ROLE OF MELANOPHAGES
Melanophages are dermal macrophages that have phagocytosed melanin, usually as a result of epidermal melanin leakage due to barrier disruption, inflammation, or chronic stimulation. They are commonly found in hyperpigmented lesions, especially in dermal and mixed-type melasma.
Unlike melanocytes, melanophages do not synthesize melanin; instead, they scavenge melanin that has translocated from the epidermis into the dermis.
Their involvement is particularly noted in dermal and mixed-type melasma, where free melanin and clusters of melanophages are commonly observed in the upper dermis. Reflectance confocal microscopy (RCM), enables non-invasive visualization of melanin distribution, supporting the histological classification of melasma subtypes.
Because dermal melanin is more persistent and less responsive to topical therapies, the presence of melanophages represents a major therapeutic barrier in chronic hyperpigmentation. Understanding their dynamics is crucial for developing more effective pigment clearance strategies, especially in recalcitrant melasma.
ROLE OF VASCULAR ENDOTHELIAL CELLS
Vascular components are increasingly recognized as active participants in pigmentary disorders such as melasma. Histological studies have consistently reported increased vascularization in lesional skin, accompanied by elevated expression of angiogenic factors such as vascular endothelial growth factor (VEGF) and increased vascular density in the upper dermis.
Endothelial cells can influence melanogenesis through paracrine signalling, particularly under conditions of inflammation or UV exposure. VEGF has been shown to modulate melanocyte function and melanin production, and some studies suggest that anti-angiogenic treatments may improve pigmentation in vascular-rich lesions.
This supports the notion that vascular remodelling is not merely a secondary feature of melasma, but may contribute to its initiation and persistence.
OTHER CELLULAR CONTRIBUTORS
Beyond the core cell types already discussed, additional actors may modulate pigmentation through more indirect but biologically relevant pathways.
Sebocytes, particularly in seborrheic areas, produce pro-inflammatory lipids and cytokines that can influence local inflammation and oxidative stress, thereby indirectly affecting melanocyte activity.
Cutaneous immune cells such as Langerhans cells and dermal dendritic cells can also shape pigmentary responses through their secretion of IL-1, TNF-α, or reactive oxygen species during immune surveillance or post-injury inflammation.
Furthermore, the skin’s neurocutaneous system, including peripheral nerves and their associated neuropeptides (e.g., substance P, CGRP), has been implicated in pigment regulation. These neuropeptides can modulate melanocyte behavior both directly and via cross-talk with keratinocytes and fibroblasts under stress or sensory stimuli.
Adipocytes also influence pigmentation through the secretion of adipokines and, more specifically, via adipose-derived stem cells, which can inhibit melanocyte proliferation and melanin synthesis through an interleukin-6 (IL-6)-mediated mechanism, highlighting their potential regulatory role in dermal-epidermal pigmentary dynamics.
While the roles of these cells remain less well-defined compared to keratinocytes or fibroblasts, growing evidence supports their involvement in complex pigmentation disorders such as melasma and post-inflammatory hyperpigmentation. Future studies will help clarify their precise functions and therapeutic potential.
ROLE OF THE SKIN MICROBIOME
Beyond resident skin cells, the skin microbiome represents a crucial regulator of pigmentation homeostasis, acting through both immune modulation and metabolic signalling.
Dysbiosis, a disruption in the microbial balance, has been associated with increased oxidative stress, low-grade inflammation, and impaired barrier function, all of which can exacerbate pigmentary disorders such as melasma or post-inflammatory hyperpigmentation.
Certain commensal bacteria may influence melanogenesis either directly or indirectly by stimulating keratinocyte-derived cytokines and oxidative mediators.
Emerging studies also suggest that UV-induced microbiome shifts can potentiate pigmentary photoresponses, particularly in melanin-rich skin where pigment dysregulation is more reactive to inflammatory triggers.
2. Cellular senescence: A hidden driver of pigmentary imbalance
While the role of inflammation and UV-induced damage in pigmentation disorders is well established, cellular senescence is now emerging as a central—but often overlooked—contributor to dysregulated pigmentation, extending beyond hyperpigmentation alone.
Senescent cells accumulate in chronologically aged or photo-exposed skin and develop a distinct secretory profile, termed the senescence-associated secretory phenotype (SASP). This comprises pro-inflammatory cytokines (e.g., IL-6, IL-8), growth factors (e.g., HGF, SCF), proteases (e.g., MMP-1, MMP-3), and oxidative stress mediators, all of which can perturb cutaneous homeostasis and influence melanogenic pathways.
Among cutaneous cells, fibroblasts are major SASP producers in aged dermis. In melasma and photoaged skin, senescent fibroblasts secrete increased levels of melanocyte-activating factors such as hepatocyte growth factor (HGF), stem cell factor (SCF), and DKK1, a Wnt pathway antagonist that may alter epidermal architecture and pigmentation balance. This contributes not only to melanocyte hyperactivity, but also to dermo-epidermal disjunction, favoring pigment deposition and retention in the dermis.
Importantly, melanocytes themselves are not immune to senescence. Senescent melanocytes exhibit increased dendricity, altered expression of melanogenesis enzymes, mitochondrial dysfunction, and impaired response to paracrine cues.
These intrinsic changes may predispose to excessive melanin synthesis, but also to hypopigmentary conditions such as vitiligo. In vitiligo, melanocyte senescence may contribute to reduced cellular resilience, oxidative stress vulnerability, and immune recognition, facilitating their clearance by cytotoxic responses.
Pigmentary disorders, whether hyper- or hypopigmentary, may share senescence as a unifying upstream mechanism, albeit through different downstream cascades. Addressing cellular senescence, particularly in fibroblasts and melanocytes, offers a promising therapeutic axis for melasma, photoaging, and pigment loss syndromes.
3. Redesigning evaluation tools: Modeling pigmentation as a system
To fully embrace this new paradigm, the evaluation of pigmentary treatments must evolve alongside our understanding of pigmentation biology. Conventional models focusing solely on melanocyte monocultures or melanin quantification no longer suffice to capture the complexity of chronic hyperpigmentation.
At QIMA Life Sciences, we address this challenge through advanced preclinical platforms that reflect the multicellular and microenvironmental dynamics of pigmentation disorders:
- 3D co-culture systems incorporating keratinocytes, melanocytes, and fibroblasts, enabling a realistic appraisal of intercellular signalling across the epidermal–dermal interface.
- Phototype-diverse cell panels to evaluate responses across a spectrum of skin tones, particularly critical when assessing pigmentary risks such as post-inflammatory hyperpigmentation (PIH).
- Senescence-enriched dermal models that simulate aging skin niches, allowing efficacy screening of senotherapeutic candidates.
- Inflammation- and vascular-stimulated skin models to probe the pigmentary effects of anti-inflammatory or anti-angiogenic treatments.
These integrated platforms support the development of mechanism-driven, phototype-adapted solutions that not only lighten visible pigmentation but restore the biological balance of the skin.
4. Conclusion: Embracing the multicellular complexity of pigmentation
Pigmentation is no longer a story of melanocytes alone.
As scientific knowledge deepens, it becomes clear that hyperpigmentation, whether linked to aging, inflammation, or environmental triggers, is the outcome of a multicellular symphony involving epidermal, dermal, immune, and vascular players. The microenvironment, influenced by senescence, inflammation, and remodeling, plays a decisive role in shaping pigment dynamics.
Understanding this complexity is not only key to deciphering conditions like melasma or post-inflammatory hyperpigmentation but also to developing more targeted, durable, and inclusive solutions. Therapies that act exclusively on melanogenesis may offer temporary results, but those addressing the broader ecosystem (fibroblast senescence, vascular crosstalk, barrier integrity) hold the promise of sustained efficacy across diverse phototypes.
This paradigm shift urges both researchers and formulators to adopt holistic strategies, rooted in advanced biology and multicellular modeling.
Written by:
Rachida Nachat-Kappes, PhD
Skin Biology Expert
Edited by:
Mara Carloni, PhD
Scientific Communications & Marketing Project Leader
Last update: 30/10/2025
Understanding how pigmentation arises from multicellular interactions and senescent skin microenvironments is essential for designing more effective, long-lasting hyperpigmentation treatments.
At QIMA Life Sciences, we provide innovative preclinical and clinical models that go beyond melanocyte monocultures to capture the true biological complexity of pigmentary disorders.
With a portfolio of phototype-representative in vitro and ex vivo models, we help you evaluate efficacy, tolerance, and pigmentation risk in a physiologically relevant way, across Fitzpatrick phototypes I to VI.
Eager to bring your skincare innovations to life?
References
- Baxter LL, Watkins‐Chow DE, Pavan WJ, and Loftus SK. A curated gene list for expanding the horizons of pigmentation biology. Pigment Cell Melanoma Res. 2019
- Prospéri M-T, Giordano C, Gomez-Duro M, Hurbain I, Macé A-S, Raposo G, and D’Angelo G. Extracellular vesicles released by keratinocytes regulate melanosome maturation, melanocyte dendricity, and pigment transfer. PNAS. 2024
- Guo H, Xing Y, Liu Y, Luo Y, Deng F, Yang T, Yang K, and Li Y. Wnt/β-catenin signaling pathway activates melanocyte stem cells in vitro and in vivo. Journal of Dermatological Science. 2016
- Wang Y, Viennet C, Robin S, Berthon J-Y, He L, and Humbert P. Precise role of dermal fibroblasts on melanocyte pigmentation. J Dermatol Sci. 2017
- Kim M, Kim SM, Kwon S, Park TJ, and Kang HY. Senescent fibroblasts in melasma pathophysiology. Exp Dermatol. 2019
- Kim JC, Park TJ, and Kang HY. Skin-Aging Pigmentation: Who Is the Real Enemy? Cells. 2022
- Nielsen VW and Thomsen SF. The role of the mast cell in pigmentation disorders. Pigment International. 2021
- Yoshida M, Takahashi Y, and Inoue S. Histamine Induces Melanogenesis and Morphologic Changes by Protein Kinase A Activation via H2 Receptors in Human Normal Melanocytes. Journal of Investigative Dermatology. 2000
- Guitera P, Li L-XL, Scolyer RA, and Menzies SW. Morphologic features of melanophages under in vivo reflectance confocal microscopy. Arch Dermatol. 2010
- Liu H, Lin Y, Nie X, Chen S, Chen X, Shi B, Tian H, Shi Z, Yu M, Zhang D, et al. Histological classification of melasma with reflectance confocal microscopy: a pilot study in Chinese patients. Skin Res Technol. 2011
- Kim EH, Kim YC, Lee E-S, and Kang HY. The vascular characteristics of melasma. Journal of Dermatological Science. 2007
- Masub N, Nguyen JK, Austin E, and Jagdeo J. The Vascular Component of Melasma: A Systematic Review of Laboratory, Diagnostic, and Therapeutic Evidence. Dermatol Surg. 2020
- Zhu J-W, Ni Y-J, Tong X-Y, Guo X, and Wu X-P. Activation of VEGF receptors in response to UVB promotes cell proliferation and melanogenesis of normal human melanocytes. Exp Cell Res. 2020
- Flori E, Mastrofrancesco A, Mosca S, Ottaviani M, Briganti S, Cardinali G, Filoni A, Cameli N, Zaccarini M, Zouboulis CC, et al. Sebocytes contribute to melasma onset. iScience. 2022
- Koike S and Yamasaki K. Melanogenesis Connection with Innate Immunity and Toll-Like Receptors. International Journal of Molecular Sciences. 2020
- Toyoda M, Luo Y, Makino T, Matsui C, and Morohashi M. Calcitonin gene-related peptide upregulates melanogenesis and enhances melanocyte dendricity via induction of keratinocyte-derived melanotrophic factors. J Investig Dermatol Symp Proc. 1999
- Egriboz O, Fehrholz M, Tsutsumi M, Sousa M, Cheret J, Funk W, Kückelhaus M, Paus R, Kajiya K, Piccini I, et al. The Melanocyte and Nerve Fiber Cross-Talk, Facilitated Also by Semaphorin-4A, Enhances UV-B-Induced Melanogenesis. Pigment Cell Melanoma Res. 2025
- Kim D-W, Jeon B-J, Hwang N-H, Kim M-S, Park S-H, Dhong E-S, Yoon E-S, and Lee B-I. Adipose-derived stem cells inhibit epidermal melanocytes through an interleukin-6-mediated mechanism. Plast Reconstr Surg. 2014
- Hu Y, Chen Y, Xu J, Luo H, Lu H, Xie B, Du X, and Song X. Dysbiosis of skin microbiome in melasma patients. Pigment Cell & Melanoma Research. 2022
- Zanchetta C, Vilanova D, Jarrin C, Scandolera A, Chapuis E, Auriol D, Robe P, Dupont J, Lapierre L, and Reynaud R. Bacterial taxa predictive of hyperpigmented skins. Health Sci Rep. 2022
- L’Orphelin J, Dompmartin A, and Dréno B. The Skin Microbiome: A New Key Player in Melanoma, From Onset to Metastatic Stage. Pigment Cell Melanoma Res. 2025
- Dong B-Q, Liao Z-K, Le Y, Jiang S, Luo L-F, Miao F, Le Poole IC, and Lei T-C. Acceleration of melanocyte senescence by the proinflammatory cytokines IFNγ and TNFα impairs the repigmentation response of vitiligo patients to narrowband ultraviolet B (NBUVB) phototherapy. Mech Ageing Dev. 2023






