Share this Technical Focus
Key takeaway:
Our in-depth characterized model of mast cells is a valuable tool for research and testing.
It provides a unique opportunity to work with primary cells, increasing clinical relevance of studies.
Mast cells are immune cells involved in inflammation, serving as a first line of defense against specific pathogens and playing a central role in allergic reactions.
Current models for studying mast cells face several limitations: immortalized cell lines undergo alterations due to long-term culture; animal-derived mast cells raise ethical concerns and offer limited translational relevance; and mast cells derived from CD34+ peripheral blood cells require prolonged culture times.
Our team developed this model by adapting a protocol previously described by Siiskonen and Scheffel (Methods Mol Biol, 2020).
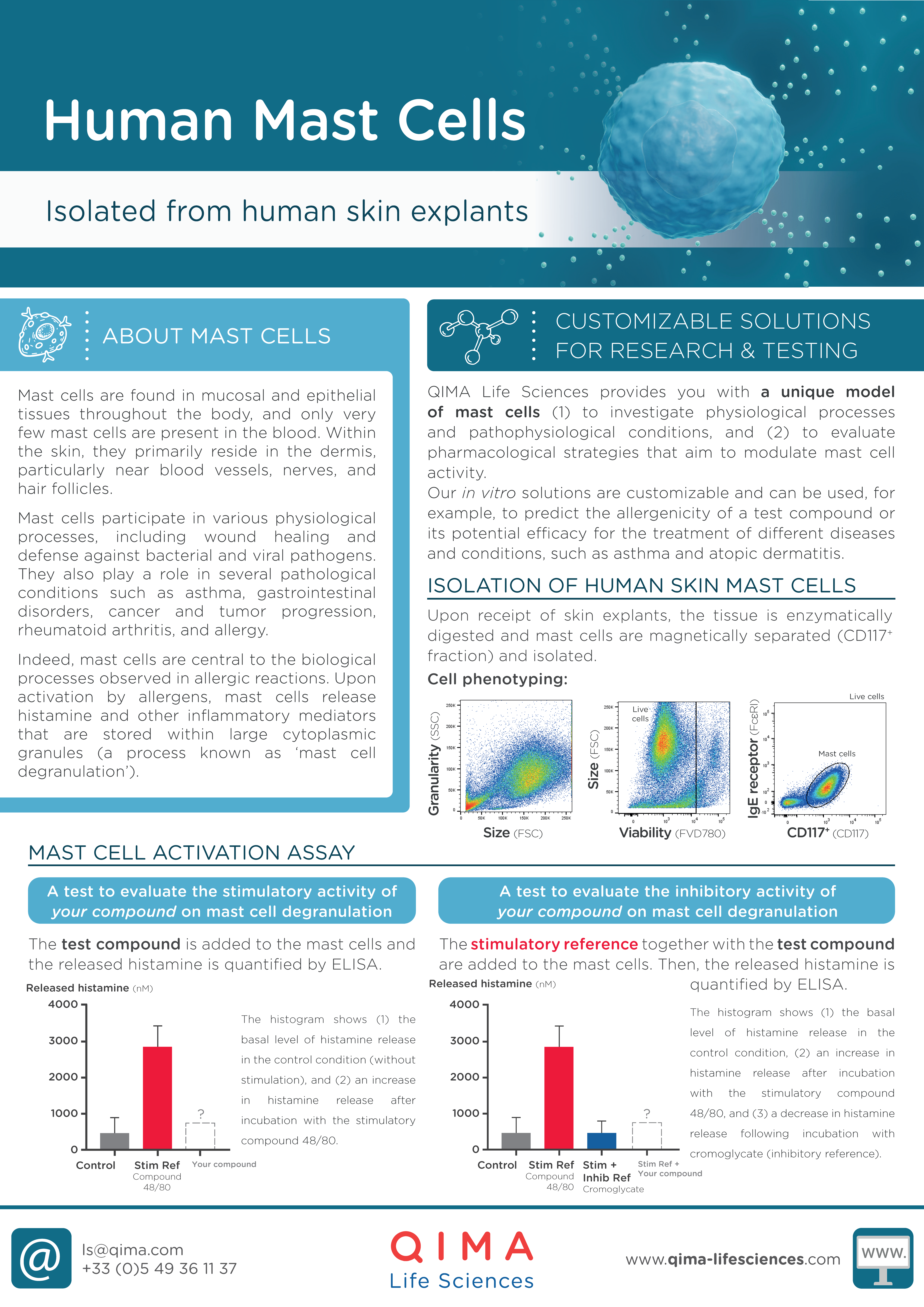
Briefly, upon receipt of skin explants, the tissue undergoes enzymatic digestion, followed by magnetic separation to isolate the CD117⁺ mast cell fraction.
Download our flyer on
Human Mast Cells
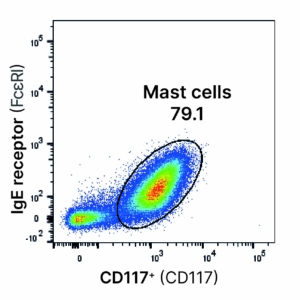
Mast cell enrichment control
Test: Cell phenotyping
Method: Magnetic cell enrichment (MACS) / Flow cytometry
Observation: Magnetic enrichment of mast cells (CD117⁺) following enzymatic digestion of skin explants resulted in approximately 80% viable mast cells.
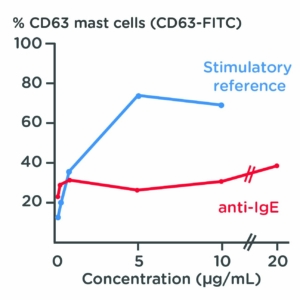
Mast cells marker analysis
Test: Cell phenotyping
Method: Flow cytometry – dose response curve
Observation: CD63, a marker of mast cell activation, was upregulated on isolated primary human mast cells following stimulation. Treatment with anti-IgE had no significant effect on CD63 expression.
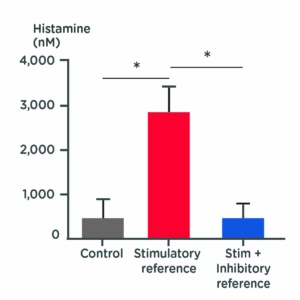
Histamine release quantification
Test: Histamine dosage
Method: ELISA test
Observation: The stimulatory compound increases histamine release from mast cells, whereas treatment with the inhibitory compound effectively inhibits histamine release in activated mast cells.
This model can be used to test new drugs or compounds aimed at modulating mast cell activity. It is also useful for identifying the risk of histamine release by a compound or formula, supporting a safety-oriented approach.
Written by:
Mara Carloni, PhD
Scientific Communications & Marketing Project Leader
Edited by:
Sabrina Hoefling, PhD
Innovation Marketing Project Leader
Request access to our
Human Mast Cells Poster