The skin, the body’s largest organ, covers the outer surface and forms a boundary with the environment. Its primary role is to act as a physical and chemical barrier, shielding the body from external aggressions. However, it also serves as an immune organ, harboring innate and adaptive immune cells that work together to eliminate pathogens after tissue damage.
However, inappropriate immune responses in the skin can result in autoimmune skin diseases (AISDs), such as pemphigus, dermatitis herpetiformis, vitiligo, and alopecia areata (AA).
What underlies these autoimmune diseases, and how is the medical community responding to them? Let’s explore this complex topic.
What Are Autoimmune Diseases?
First of all, it is essential to introduce the concept of autoimmune diseases (ADs) – complex pathologies in which the immune system attacks the very body it is meant to protect. This dysfunction stemming from an imbalance between effector and regulatory immune responses can result in chronic and potentially life-threatening inflammatory disturbances, in addition to having a severe impact on the quality of life. ADs trigger aberrant reactivity of B-cells and T-cells against normal components of the body, specifically targeting one or more autoantigens.
ADs encompass a broad spectrum of conditions, which can be systemic (e.g., systemic lupus erythematosus) or organ-specific (e.g., vitiligo, primarily affecting the skin). Currently, over 80 ADs have been described.
The clinical features of ADs are highly varied, ranging from acute, life-threatening organ failure to subtle laboratory abnormalities that can easily be overlooked. The main immunological manifestation of ADs is the production of autoantibodies, which can be present many years before the onset of clinical or laboratory symptoms of the condition, a phase known as preclinical autoimmunity.
Although the etiology of ADs is complex and largely unknown, genetic and environmental factors can contribute to their pathogenesis. Genetic predisposition to autoimmunity is intricate, often involving several genes that regulate the role of immune cell communities. Less commonly, autoimmunity can result from single-gene mutations that disrupt major regulatory pathways. Infection appears to be a common trigger for ADs, although the microbiome can also play a role in their pathogenesis.
What are autoimmune Skin Diseases (AISDs)?
In this article, we pay particular attention to autoimmune skin diseases (AISDs), which arise when the immune system mistakenly attacks normal skin cells, resulting in inflammation and tissue damage. These conditions impose a major burden of global disease and many currently lack effective therapies.
Generally, skin autoimmunity can occur as a consequence of different types of events such as an impaired microbiome, inherited dysfunctional immunity, antigens activating innate immunity, epigenetic changes, gender-based predisposition, and the effects of antigens either as neoantigen or through molecular mimicry and epitope spreading.
This article highlights three common skin disorders associated with autoimmune reactions: vitiligo and alopecia areata (AA), which are T-cell-mediated autoimmune skin diseases, and psoriasis, classified as both T-cell-mediated autoimmune and autoinflammatory skin disease, making it a special case.
Examples of Autoimmune Skin Diseases
Vitiligo
Vitiligo is a common depigmenting skin disorder, classified as an AD. Its prevalence has been estimated ranging from 0.5% to 2% of the population worldwide, affecting both adults and children, without any significant difference in prevalence due to sex, ethnicity, skin types or geographic region. The disease typically occurs in individuals before the age of 30 years.
Clinically, vitiligo is marked by the selective loss of melanocytes, which in turn results in the dilution of pigment in the affected cutaneous areas. A typical vitiligo lesion can be defined as a completely amelanotic, non-scaly, chalky-white macule with distinct margins.
The two major forms of the condition, recognized by an international consensus in 2011, are nonsegmental vitiligo and segmental vitiligo. These subtypes differ in their clinical manifestations (the onset of the first cutaneous lesions, their location and expanse, the coexistence of concomitant ADs, and the natural progression of the dermatosis), as well as in their etiologies. Vitiligo commonly affects hyperpigmented areas such as the face (periorificial), dorsa of the hands, nipples, axillae, umbilicus, and sacral, inguinal, and anogenital regions, surrounded by normal skin. On the extremities, it favors the elbows, knees, digits, and flexor wrists.
The pathogenesis of vitiligo is multifactorial, combining multiple intertwined components including environmental triggers, genetic predisposition, metabolic changes, increased oxidative stress, abnormal immune and inflammatory response, and melanocyte detachment mechanisms. Both innate and adaptive arms of the immune system appear to play a role.
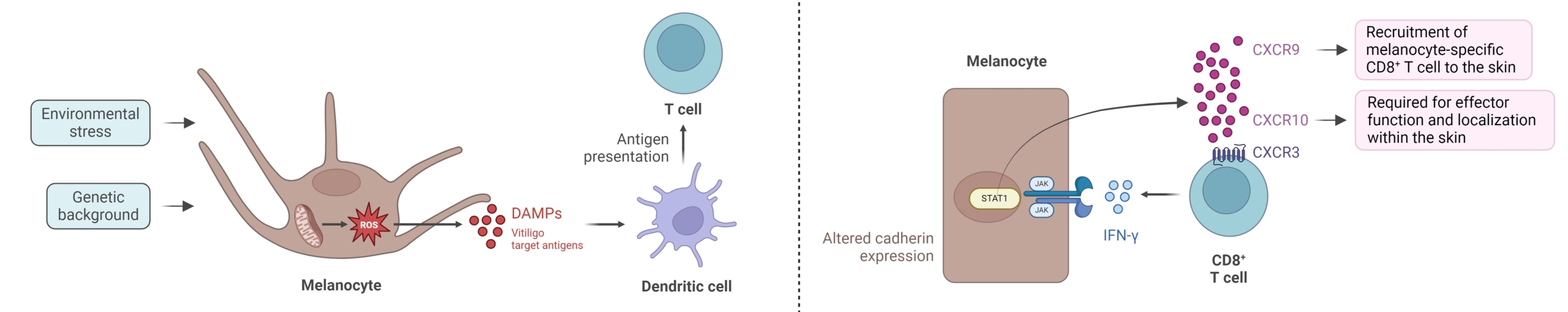
Key mechanisms involved in vitiligo pathogenesis, adapted from Bergqvist et al, 2020. (Figure created with BioRender)
Individuals with vitiligo display impaired mitochondrial functionality, leading to an increased generation of reactive oxygen species (ROS). Oxidative stress disrupts the role of membrane lipids and cellular proteins in melanocytes, and contributes to autophagy defects and Nrf2 pathway impairment.
ROS overproduction also stimulates the unfolded protein response and causes melanocytes to produce exosomes delivering autoantigens to neighboring dendritic cells (DCs), which undergo maturation into effective antigen-presenting cells (APC). Subsequently, cytokine- and chemokine-mediated activation of Th17-cells and dysfunction of regulatory T-cells (Treg) occur. The CD8+ T-cells in vitiligo lesions secrete multiple cytokines such as TNF or IFN-γ, the latter playing a central role in the progression of vitiligo. Binding of IFN-γ to its receptor IFN-γR stimulates the JAK-STAT pathway, thus contributing to the immunopathogenesis of vitiligo. This activation induces the secretion of CXCL9 and CXCL10 in the skin. Through the cognate receptor CXCR3, CXCL9 promotes the massive recruitment of melanocyte-specific CD8+ T-cells into the skin but with no effector action, while CXCL10 is required for their localization in the epidermis where melanocytes reside and for their effector function, which enhances inflammation via a positive feedback loop. Moreover, IL-15 plays a key role in sustaining autoreactive resident memory CD8+ T-cells that are responsible for relapse of disease, in vitiligo pathogenesis.
The main emerging therapies for vitiligo that are at the most advanced stages of development and are associated with the most major pathogenic pathways include the tyrosine kinase inhibitors (e.g., JAK1, JAK2, JAK3, TYK2), anti-IL-15, anti-IFN-α, Mc1R agonist, IL-2 mutein and mutant HSP70.
Alopecia Areata
Alopecia areata is a complex autoimmune condition which clinically manifests by a transient, non-scarring hair loss (on the scalp or any hair-bearing body site) with preservation of the hair follicle. Alopecia areata presents a lifetime incidence of about 2% worldwide, and affects both sexes equally and shows no significant racial predominance. While this condition might occur at any age, most cases arise before the age of 40 years, with an average age of onset between 25 and 36 years.
Several subtypes of alopecia areata with distinct presentations have been described. The most common subtype is a small round or patchy bald lesion (patchy alopecia areata), typically on the scalp, that can progress to total loss of scalp hair only (alopecia totalis) and total hair loss of the entire body (alopecia universalis).
The exact etiopathogenesis of alopecia areata is not yet fully understood and several factors, such as genetic predisposition, autoimmunity, and environmental triggers (including emotional or physical stress) contribute to the disease.
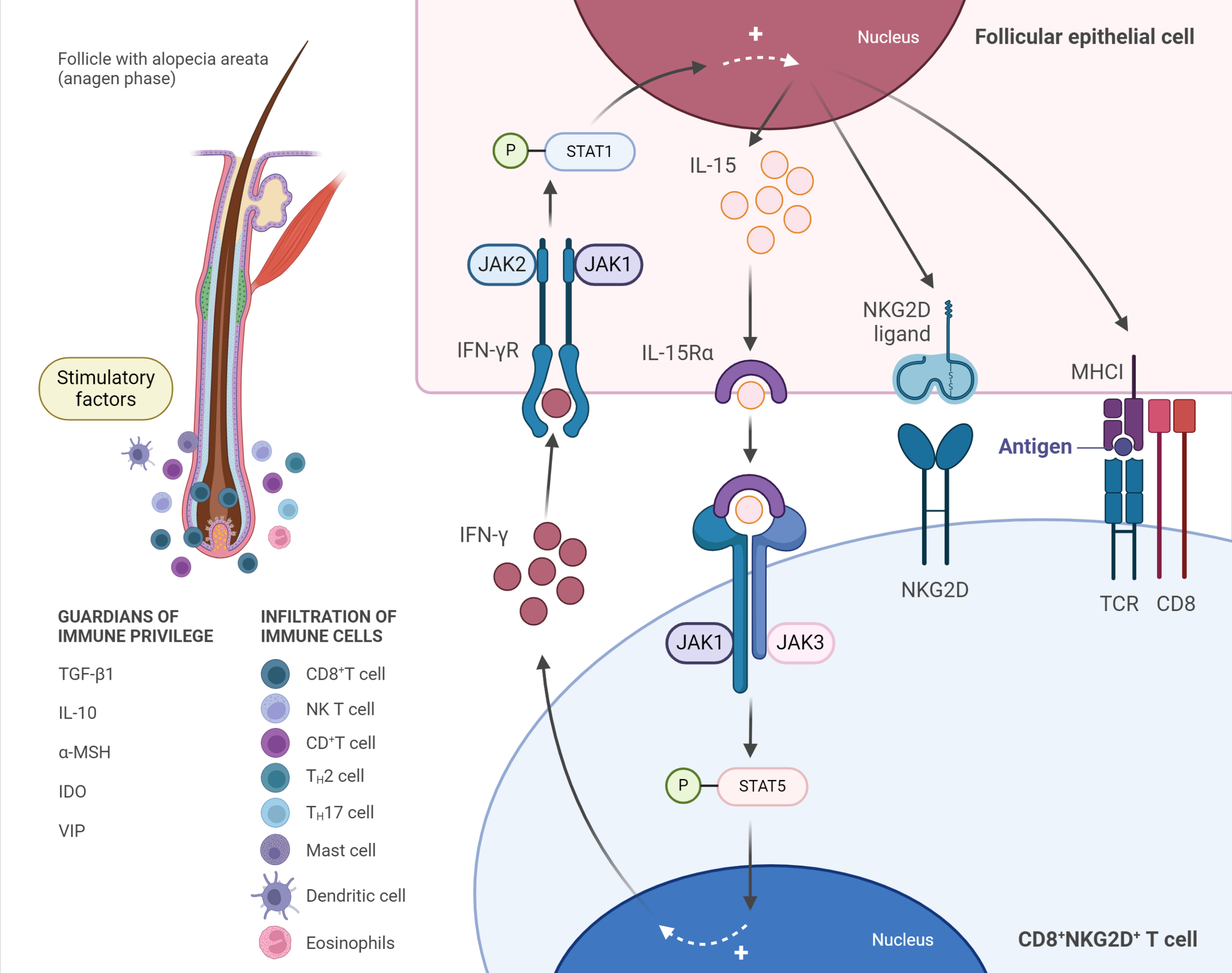
Immunopathogenesis of alopecia areata (AA), adapted from Zhou et al, 2021. (Figure created with BioRender)
Anagen-stage hair follicles are considered an ”immune privileged” site from the level of the bulge downwards to the bulb, relatively preventing autoimmune responses against autoantigens expressed during this stage. A breakdown of anagen hair bulb immune privilege is a major precondition for the development of AA. Increasing exposure of anagen hair follicle-associated autoantigens and loss of the immune privilege site may result from local increased secretion of INF-γ, cytokines and chemokines (e.g., IL-15, IL-2, CXCL2), upregulation of NKG2D ligands, and MHC I and MHC II molecules, besides the decrease of the local immunosuppressant molecules (”immune privilege guardians”) such as TGF-β1, IL-10 and α-MSH. In AA, stimulatory factors may activate CD8+ NKG2D+ T-cells, which were shown to be necessary and sufficient for the induction of the disease in mouse models, and produce IFN-γ through JAK1 and JAK3 pathways.
Stimulated CD8+ NKG2D+T-cells secrete IFN-γ that binds to its receptor IFN-γR on follicular epithelial cells, inducing IL-15 production by these cells via JAK1 and JAK2. Then, IL-15 binds to its receptor IL-15Rα on CD8+ T-cells, further stimulating IFN-γ production through JAK1 and JAK3 pathways, which amplify the positive feedback loop. IFN-γ promotes the breakdown of hair follicle immune privilege, which results in the exposure of autoantigens to CD8+NKG2D+ T-cells and facilitates the autoimmune attack on hair follicles. Simultaneously, other immune cells, including DCs, CD4+ T-cells, NK T-cells, mast cells, and eosinophils, accumulate around hair bulbs and attack them in a characteristic “swarm of bees” pattern.
Psoriasis
Plaque psoriasis, also known as psoriasis vulgaris, is marked by sharply demarcated, erythematous scaly patches or plaques that can occur on any part of the body, although commonly affected areas are the scalp, the trunk, the intergluteal cleft, and the extensor surfaces of the limbs (elbows and knees).
It affects an estimated 125 million people globally and approximately 2-4% of the population in western countries. However, its prevalence varies both geographically and among different ethnic groups within the same region. Although psoriasis can occur at any age, its onset typically follows a bimodal distribution with peaks at 18 to 39 years and also at ages 50 to 69 years.
Clinical features of psoriasis are varied and depend on the psoriasis variant. Psoriasis variants include guttate psoriasis, erythrodermic psoriasis, pustular psoriasis, inverse psoriasis and especially plaque psoriasis which is the most common one, accounting for approximately 80-90% of psoriasis cases.
The pathogenesis of psoriasis is complicated and multifactorial, involving genetic, immunological, and environmental contributions, and is not completely elucidated. In the early stages of psoriasis pathogenesis, a range of cell types, including keratinocytes, natural killer (NK) T-cells, macrophages and plasmacytoid DCs, secrete various cytokines (e.g., TNF-α, IFN-α, IFN-γ, IL-1β) that stimulate myeloid DCs. These activated myeloid DCs secrete excessive pro-inflammatory cytokines (IL-12, IL-23, and TNF-α), which in turn drive the differentiation of naive T-cells into Th1, Th17 and/or Th22-cells. Th1-cells release IFN-γ and TNF-α, Th-22 cells produce IL-22, and Th17-cells secrete IL-17, IL-22 and TNF-α to potentiate disease pathogenesis.
Among these pathways, IL-23-driving activation of the Th17 pathway is considered central in the psoriasis pathogenesis. IL-23 signaling is mediated intracellularly through TYK2, JAK2 and STAT3, resulting in transcription of key inflammatory mediators. This cytokines cascade leads to an inflammatory cascade that results in uncontrolled keratinocyte proliferation, increased expression of angiogenic mediators and endothelial adhesion molecules, heightened dermal vascularity, and infiltration of multiple immune cells into affected skin.
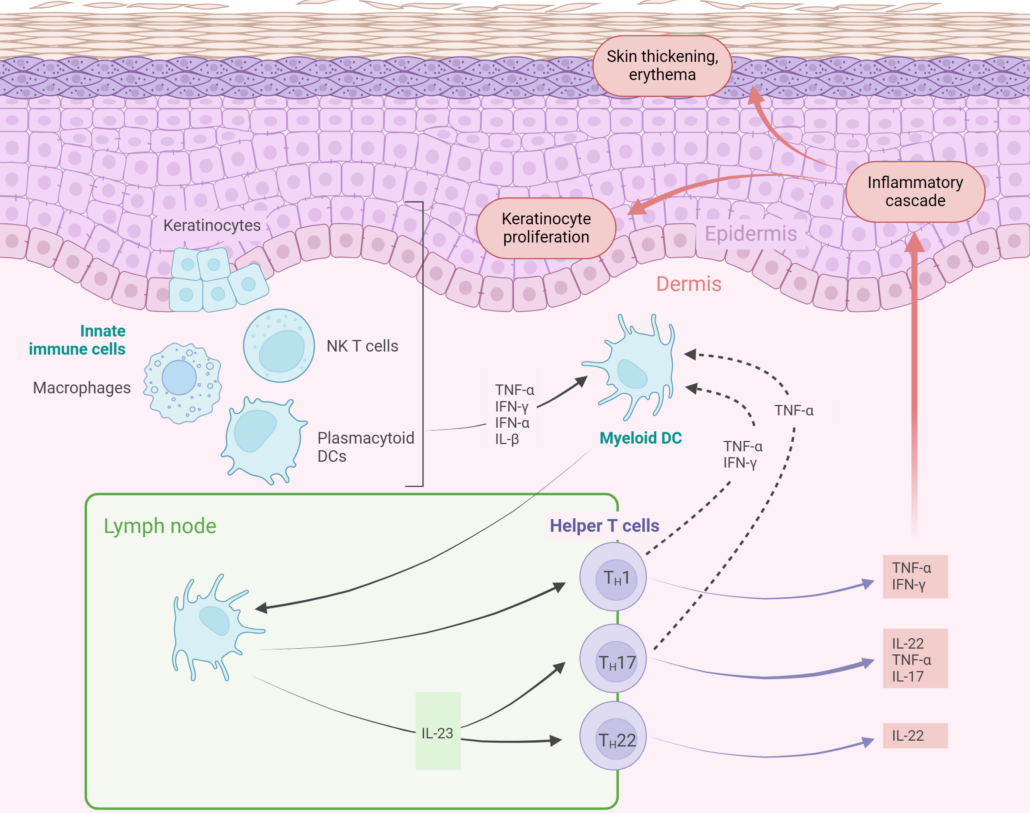
Pathophysiology of psoriasis (Figure reated with BioRender)
Download our flyer
Vitiligo
Interested in
vitiligo clinical trials?
> Discover Vitil-IA®

Watch our Webinar Replay
Download our brochure
Alopecia areata
You may also be interested in
Male & Female Pattern Hair Loss
Download our brochure
Current Therapeutics and Research
Conventional therapies for AISDs including topical treatments (e.g., corticoisteroids, calcineurin inhibitors, vitamin D analogs, keratolytics), systemic treatments (e.g., methotrexate, cyclosporine, acitretin), and even phototherapy have been used for many years to treat some AISDs to alleviate clinical signs, improve quality of life, and prevent disease progression. As side effects, treatment resistance, long-term safety concerns, disease relapse or high costs associated with some therapies limit their widespread use and efficacy. Besides the variability in treatment response among individuals, there has been a need to identify new, more effective and tailored treatments to address the specific needs of these patient populations.
Advances in molecular understanding of AISD pathogenesis has spurred the development of new more targeted therapies. Current therapeutic strategies for treating AISDs focus on targeting and inhibiting the excessive overactivity of the effector arm of immunity, including cytokine and chemokine blockade, cellular depletion, intracellular signaling blockade and costimulatory blockade. This approach has proven effective across a wide variety of AISDs through several recently approved drugs. A new wave of novel drugs following this treatment paradigm are expected to be clinically available in the coming years. In addition, in certain cases, their combination with conventional treatments can show better outcomes than monotherapy.
Although this therapeutic approach targeting the effector arm of immunity can successfully halt disease with high therapeutic efficacy and safety, especially as compared to broad immunosuppressants in certain cases, it rarely results in long-term remission. Hence, unmet medical needs for safe, effective, and durable therapies of AISDs persist.
In this respect, the emergence of therapeutic approaches targeting the regulatory arm of the immune system holds great promise, and include cellular therapy with regulatory T-cells (Treg) and chimeric autoantibody receptor (CAAR) T-cells, cytokine therapy with low-dose IL-2, immune checkpoint stimulation, tolerogenic vaccines and microbiome biotherapy. Although no licensed drugs are available to date, multiple candidates are currently in clinical trials, and this emerging paradigm should offer a new set of therapies in the future for difficult-to-treat AISDs.
| Approach | Target | Drug | Disease | Status |
| Cytokine & Chemokine blockade | TNF-α | Etanercept, Adalimumab, Certolizumab pegol, Infliximab | Psoriasis | Approved |
| IL-17 | Etanercept, Adalimumab | AA | Clinical trial | |
| Bimekizumab, Secukinumab, Ixekizumab | Psoriasis | Approved | ||
| Secukinumab | AA | Clinical trial | ||
| IL-17Rα | Brodalumab | Psoriasis | Approved | |
| IL-12/IL-23 | Ustekinumab | Psoriasis | Approved | |
| AA | Clinical trial | |||
| IL-23 | Guselkumab | Psoriasis | Approved | |
| Tildrakizumab-asmn | Psoriasis | Approved | ||
| Risankizumab | Psoriasis | Approved | ||
| IL-4Rα/IL-13 | Dupilumab | Psoriasis | Approved | |
| IFNAR1 | Anifrolumab | |||
| IL-15 | AMG 714 | Vitiligo | Clinical trial | |
| Cellular Depletion | CD2 | Alefacept | Psoriasis, AA | Clinical trial |
| Signaling Blockade | JAK1 | Abrocitinib | Psoriasis | Clinical trial |
| Povorcitinib | Vitiligo | Clinical trial | ||
| JAK1/2 | Baricitinib | AA | Approved | |
| Ruxolitinib | Vitiligo | Approved | ||
| Deuruxolitinib | AA | Approved | ||
| JAK1/2/3 | Tofacitinib | Psoriasis, AA, vitiligo, DM, CLE | Clinical trial | |
| JAK1/TYK2 | Brepocitinib | Psoriasis, AA, vitiligo | Clinical trial | |
| JAK3/TEC | Ritlecitinib | AA | Approved | |
| JAK1/2/TYK2/SYK | Cerdulatinib | Vitiligo | Clinical trial | |
| TYK2 | Deucravacitinib | Psoriasis | Approved | |
| PDE-4 | Roflumilast, Apremilast | Psoriasis | Approved | |
| Aryl hydrocarbon receptor-modulating agent | Tapinarof | Psoriasis | Approved | |
| Mc1R | Afamelanotide (α-MSH analog) | Vitiligo | Clinical trial | |
| Costimulatory Blockade | CD80/CD86 | Abatacept | AA | Clinical trial |
| CD11a | Efalizumab | Psoriasis | Approved | |
| AA | Clinical trial |
Therapeutic approaches targeting the effector arm of the immune system in AISDs (modified from Vesely, 2020). The list is not exhaustive.
Market Overview of the AISD Treatments
In addition to a steady increasing prevalence of AISDs, the market for AISD therapeutics is expected to continue expanding. This growth is driven by several factors, including heightened awareness of AISDs among the public and healthcare professionals, increasing research and development, introduction of innovative treatments, companies operating on the dermatology drugs market teaming with other pharmaceutical firms, among other reasons.
The demand for more targeted and specific treatments based on biologics and small molecules, in combination or alone, aimed at targeting effector and regulatory arms is anticipated to increase due to their potential effectiveness in halting initiation and/or progression of the disease, achieving disease control with longer-lasting benefits, managing severe symptoms and reducing relapses, with a potentially acceptable, or even better, safety profile than current treatments. These new treatment approaches are thus expected to gain market share.
Research Outlook
Despite the advancements in understanding the underlying pathogenic mechanisms and the development of novel, more targeted treatment approaches (e.g., cytokine- and chemokine-inhibitor, signaling blocker), several drawbacks persist such as side effects of therapies, treatment resistance, long-term safety concerns, disease relapse, variability in therapeutic response among individuals, and the high cost of treatments, which further limit long-term efficacy, highlighting the need for new approaches. Emerging trends and future directions in AISD treatment hold promise for improved outcomes.
These innovative strategies include (i) the development of novel treatments targeting new pathways and/or the regulatory arm of the immune system (e.g., cellular therapy) with a potentially enhanced specificity for the cutaneous autoimmune ecosystem, (ii) the exploration of combination therapies to enhance efficacy – including reducing relapses – and to minimize side effects, and (iii) personalized therapeutic approaches. In addition, (iv) a better understanding of immune mechanisms could not only improve therapeutic options but also prevent the onset of these diseases through more targeted prevention strategies.
Looking forward, the discovery and development of new therapies for AISDs will hinge on interdisciplinary collaboration and technological advancements. This will help improve outcomes and allow a better quality of life for individuals living with these chronic skin conditions. Furthermore, while the current progress shows tremendous promise, collaboration among researchers, clinicians, and industry stakeholders – including clinical research organizations (CROs) – is crucial to translate these research findings into clinical practice.
In this respect, CROs play a pivotal role in this process by supporting the entire drug development pipeline, from the early stages of drug discovery and preclinical development to the final stages of clinical trials and regulatory approval. Their expertise, tools, and frameworks are crucial for executing successful development stages, saving clients time and resources, while enhancing the quality of their research endeavors.
Earger to learn more about how we can support your research?
Written by:
Philippe Gentine, PhD
Life Sciences R&D Expert
Edited by:
Mara Carloni, PhD
Scientific Communications & Marketing Project Leader
References
About autoimmune diseases
Pisetsky DS. Pathogenesis of autoimmune disease. Nat Rev Nephrol. 2023 Aug;19(8):509-524.
About autoimmune skin diseases
Ujiie H. Regulatory T cells in autoimmune skin diseases. Exp Dermatol. 2019 Jun;28(6):642-646.
About Alopecia areata
Yu L, Lu Z. Linear alopecia areata. JAAD Case Rep. 2018 Nov 14;4(10):1072-1073.