WHAT YOU WILL LEARN IN THIS ARTICLE:
- What Are The Hallmarks of Cancer?
- The Early Stages of Cancer Drug Discovery & Deveolpment
- In Vitro Assays for Cancer Research
In this article, you will learn about some of the most utilized in vitro assays and techniques used to examine the hallmark features of cancer, i.e., the phenotypic traits that enable tumor growth and metastatic dissemination, in the context of anti-cancer drug discovery and development.
What Are The Hallmarks of Cancer?
By definition, cancer is a genetic disease and is defined by the presence of cancer cells. These cells initiate tumors and drive their progression forward.
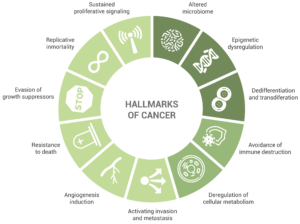
The review “The Hallmarks of Cancer”, published in 2000 by Hanahan and Weinberg, was a fundamental contribution to the study of cancer. This publication conceptualized the biology of the disease by describing its hallmarks; the review enumerated particular traits found in almost every type of cancer that enable tumor growth (in the first place) and metastatic dissemination (in the later stages).
Initially, the accepted hallmarks of cancer were six, but only some years after, the authors extended this number to eight:
-
Sustained proliferative signaling. This capability is acquired by cancer cells in a number of different ways, such as the production of growth factors ligands that will serve an autocrine stimulation or the increase in the number of receptor proteins.
-
Evasion of growth suppressors. An example of this is the fact that cancer cells do not present contact inhibition. Indeed, unlike normal cells, cell-to-cell contacts do not limit cancer cell proliferation.
-
Resistance to death, including terminal differentiation program, senescence, or apoptosis. Cancer cells display several strategies to circumvent – or at least limit – apoptosis. One of the most common is the elimination of a key damage sensor protein, p53. However, the multiplicity of mechanisms by which cancer cells avoid apoptosis is very large (as it is the diversity of signals inducing this cell death mechanism).
-
Replicative immortality. It has been long clear to researchers that macroscopic tumors result from a great replicative potential. This in contrast to most normal cell types in the body, which can only have a limited number of division cycles.
-
Angiogenesis induction. The creation of new blood vessels helps sustain cancer cells and provide them with the nutrients and oxygen required for survival. This is enabled by chronically activated angiogenesis but also by proangiogenic signals.
-
Activating invasion and metastasis. Starting with local invasion, cancer cells are extravasated into the blood and lymphatic vessels. In some occasions, cancer cells escape these vessels into the parenchyma of distant tissues, forming micrometastases, which will later colonize the new location.
-
Deregulation of cellular metabolism.
-
Avoidance of immune destruction. Cells from the immune system display a role of immune surveillance, by which incipient cancer cells should be recognized and eliminated. Yet, the fact that tumors do appear shows that cancer cells somehow manage to avoid or limit immunological killing.
In light of the results of the last ten years of research in the cancer field, Senga and Grose proposed in a recent review the addition of new emerging hallmarks, including dedifferentiation and transdifferentiation, epigenetic dysregulation, and altered microbiome.
The Early Stages of Cancer Drug Discovery & Development
One of the very first steps in cancer drug research is evaluating if the new drug works – in other words, whether it has the desired effect. Typically, we tend to think that the desired effect for a cancer drug necessarily means causing a stall in the division of cancer cells, but it is important to keep in mind that the desired effects can be very diverse and involve different cell types, not only cancer cells. An example of immune cells as targets for cancer drugs is, for instance, the reprogramming of TAMs (tumor-associated macrophages, present in the tumor microenvironment), into an anti-tumor phenotype (M1 type).
Cancer research still relies greatly on in vitro analysis. Although in vivo testing of cancer drugs remains mandatory before clinical trials on humans, in vitro is still key to high throughput screening at the early stages of research and development. These analyses are of great utility in enabling the selection of only a small number of drugs for further preclinical assessment.
While 3D models better recreate the complexity of tumors, 2D models remain the basis of high-throughput screening of large libraries of compounds. 2D in vitro cell cultures are easy to implement, do not require animal use, and are cost-effective and robust. Therefore, they provide an initial platform for cancer drug discovery approaches.
In the case where cancer cells are the desired target of the drug, a set of compounds will be tested on human tumor cell lines or primary cultures. Generally, cell lines are preferred since they provide an unlimited supply of material that is easy to propagate. Although no in vitro model can fully mimic reality, the choice must consider the advantages and limitations of each, guided by the aim of the study. Wherever possible, cell lines should resemble the tumor subtype as much as possible.
The tumor microenvironment is crucial for a tumor’s evolution and, therefore, for cancer studies. One way to bring in vitro cell culture conditions closer to the in vivo situation is to coculture cancer cell lines with cellular representatives of the tumor microenvironment, such as macrophages or fibroblasts.
Once one or several compounds have proven effective in the first preclinical stages, testing moves to the next preclinical steps. For more information about the preclinical stages of the drug discovery and development process, please read our article on In Vitro Pharmacology for Drug Discovery & Development.
Discover other blog articles
In Vitro Assays for Cancer Research
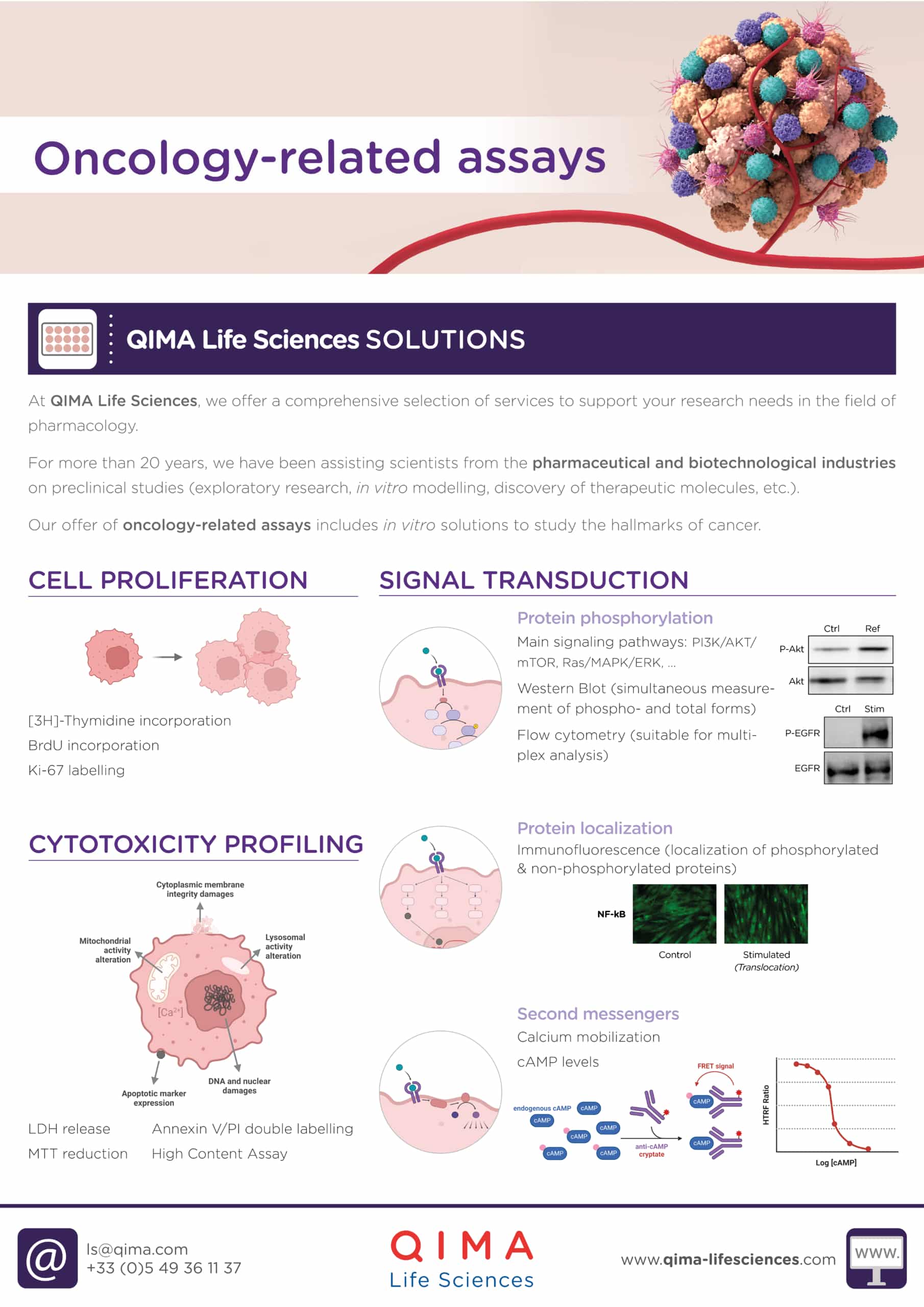
1. Cytotoxic Profiling
There are several in vitro assays to assess the cytotoxicity of a compound on tumor cells. These have been categorized into four groups: (a) dye exclusion assays, (b) colorimetric assays, (c) fluorometric assays, and (d) luminometric assays.
Choosing the appropriate assay is crucial as there are various cell death mechanisms that exist. These mechanisms differ in terms of the molecules involved and cellular changes, as well as their speed. Thus, it is important to clearly determine the cell death mechanisms that we want to study before selecting the appropriate method. For example, some assays only provide information on the late stages of cell death by measuring cell membrane disruption, while others can detect earlier cellular processes such as replication stall or DNA damage that occur before cell membrane integrity is compromised.
Four of the most common assays to assess the cytotoxicity of a compound on tumor cells are the following:
LDH RELEASE
LDH is an acronym for lactate dehydrogenase, which is a cytosolic enzyme. The LDH assay works on the principle that when a cell’s viability is compromised, the plasma membrane becomes leaky and the LDH, which is typically present in the cell cytoplasm, is released into the cell culture medium. This is a quantitative colorimetric method that enables us to assess the cytotoxic effect of a compound by measuring the damage provoked to cells. In this assay, the released LDH is coupled to an enzymatic reaction that will result in the reduction of NAD to NADH. In a second step, the NADH will reduce a tetrazolium salt, which will produce a red-colored product, measurable by a spectrophotometer. This assay is useful for assessing the late stages of apoptosis.
MTT REDUCTION
The MTT reduction assays is a colorimetric assay that is used to determine cell viability. MTT is a colorless cell-permeable compound that can be reduced to a purple product by mitochondrial enzymes (such as succinate dehydrogenase) once inside the cells. Thus, the assay determines cell viability through the determination of the mitochondrial function of cells since the amount of metabolic activity directly correlates with the amount of viable cells. The quantification can be performed using a spectrophotometer. This assay is also useful for assessing the late stages of apoptosis.
ANNEXIN V / PI DOUBLE STAINING
This assay is best suited for non-adherent cells, i.e., cells in suspension. It is also helpful to distinguish necrosis from apoptosis. Labeling of PI and subsequent flow cytometry analysis: apoptosis. This assay is particularly helpful when working with non-adherent cells such as immune cells. Phosphatidylserine is a lipid that is normally located on the intracellular side of the cell membrane. However, in the early stages of apoptosis, phosphatidylserine translocates to the cell surface. Annexin V is a protein that binds to phosphatidylserine, but this only occurs when the lipid is found on the outside of cells. PI is a staining that intercalates between DNA bases. In a cell culture, PI can only access cell DNA when the cell is permeable, making it an indirect marker of membrane integrity. According to the signals presented by these fluorophores, it is possible to distinguish between different cases: PI–V– (non-apoptotic cells, intact membrane and normal membrane structure) PI–V+ (early-apoptotic cells, intact membrane but altered membrane proteins), and PI+V+ (late-apoptotic and necrotic cells, membrane disruption and alteration).
HIGH CONTENT ASSAY
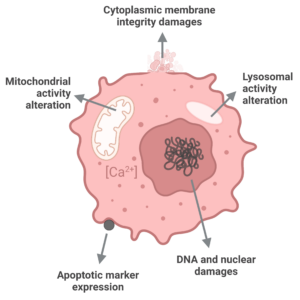
This high content assay is based on multiparameter analysis (cell number, nuclear size & intensity, cytoplasmic membrane potential, mitochondrial membrane potential, and intracellular calcium level) using fluorescent dies and imaging read-out.
2. Cell proliferation
Uncontrolled proliferation is a well-known feature of cancer cells. Cell proliferation assays are commonly used to determine the direct antiproliferative effects of test drugs on these cells. These assays can be conducted with or without the use of radioactivity.
[3H]-THYMIDINE INCORPORATION
This assay detects newly synthesized DNA in cells. Cells in culture are mixed with a radioactive version of the thymidine base. During mitosis, newly replicated DNA strands will incorporate the radioactive base, whereas non-replicative cells will not incorporate radioactivity. Radioactivity measured with a scintillation counter is proportional to the proliferation rate.
BrdU INCORPORATION
In this assay, cells in culture are mixed with a thymidine-analogue, 5-bromodeoxyuridine (BrdU). During mitosis, newly replicated DNA strands will incorporate BrdU during, whereas non-replicative cells will not incorporate it. First, a BrdU-specific monoclonal antibody is added, which will bind to the incorporated BrdU. A secondary fluorescent antibody is then added to produce a signal proportional to the DNA synthesis rate. Concentration-response curves can be generated to determine the drug’s efficacy and potency.
Ki-67 LABELLING
Ki-67 is a non-histone nuclear protein expressed at all stages of the cell cycle, except for G0, making it an excellent marker for cell proliferation. Ki-67 immunolabelling of tissue biopsies provides information regarding the proliferation state of cells in a given tissue, which can be followed by histological analysis.
3. Signal transduction
Cancer cells frequently acquire mutations in genes that regulate in signaling pathways. Consequently, some of these pathways become deregulated, promoting the development and progression of cancer. As a result, many of the molecular targets identified in oncology are kinases or receptors that exhibit increased expression in cancer cells.
Target engagement, i.e., the binding of a drug to its target, can be analyzed through direct or indirect methods. Direct methods typically involve the use of energy transfer techniques to measure interaction between the drug and the target, whereas indirect methods can involve quantifying the effects on protein phosphorylation, protein localization, and second messenger synthesis and activity.
PROTEIN PHOSPHORYLATION
Several techniques can be used to study protein phosphorylation, including ELISA, western blot analysis, and flow cytometry.
PROTEIN LOCALIZATION
Protein distribution and dynamics within cells can be studied by fluorescence microscopy, which involves labeling the protein of interest with antibodies before imaging. This technique allows researchers to visualize the translocation of important nuclear factors, such as NF-kB, in response to signaling cascades.
SECOND MESSENGERS
The selection of an appropriate assay method depends on the specific molecule of interest. For example, the cAMP assay is typically performed using Homogeneous Timer-Resolved FRET (HTRF), while the calcium mobilization assay involves the use of a calcium-sensitive fluorescent dye that can penetrate cells. Both methods allow for the quantification of second messengers via fluorescence measurement.
4. Vascularization assessment
In growing tumors, new tumor cells can die due to a lack of local oxygen and nutrients. To survive and grow, tumors vascularize by creating new blood vessels. This process, known as tumoral angiogenesis, plays a major role in cancer growth and metastasis, and largely depends largely on VEGF.
Some of the most frequently utilized in vitro assays to evaluate vascularization include the following:
VEGF RELEASE
VEGF levels in culture supernatants or tumor biopsies can be measured by ELISA.
ADHESION PROTEINS EXPRESSION
Aberrant expression of adhesion molecules has been associated with increased invasiveness and metastasis of cancer cells. The protein levels can be analyzed through various techniques, such as fluorescence microscopy.
CELL TUBE FORMATION
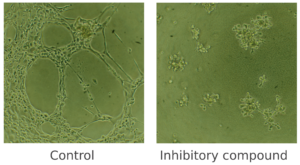
This assay is a useful method for measuring the ability of endothelial cells to form capillary-like tubules. Under pro-angiogenic conditions, endothelial cells align and form tubules, a process that can be observed through phase-contrast inverted microscopy or fluorescence microscopy if the cells are fluorescently labeled. Conversely, under angiogenic-inhibitory conditions, the number of nodes, meshes, and tubes, as well as their length, will be significantly lower.
Figure 1. Cell tube formation assay. In this example, Human Microvascular Endothelial Cells (HMVEC) were cultured in vitro and were treated with an inhibitory compound (on the right), which prevented any cell tube formation. An untreated control (on the left), to which no compound was added, was also included.
Discover our oncology-related assays
5. Immune cells
The understanding of tumors’ biology can no longer be limited to the study of cancer cells alone. Therefore, researchers also employ in vitro assays to investigate other cells present in the tumor microenvironment, including immune cells. These assays are diverse and could be the subject of a whole different article. However, we provide a brief list below, which is not exhaustive. Such assays examine the immuno-modulation effects of drugs, either in isolated immune cells or in whole blood assays.
Flow cytometry and immunoassays methods are combined to analyze:
- Cell activation
- Phagocytosis
- Inflammatory markers expression
- Cell phenotype
- Chemotaxis and migration
- Cell differentiation or polarization
You may also be interested in
Immune Response Testing
References
Hanahan D. & Weinberg R.A. The Hallmarks of Cancer. Cell (2000).
Hanahan D. & Weinberg R.A. The Hallmarks of Cancer: The Next Generation. Cell (2011).
Hanahan D. Hallmarks of Cancer: New Dimensions. Cancer Discovery (2022).
Senga S.S. & Grose R.P. Hallmarks of cancer – The new testament. Open Biology (2021).
Find a Research and Testing Program that Suits Your Needs
At QIMA Life Sciences, we offer a comprehensive selection of services to support your research needs in pharmacology. Our offer of oncology-related assays includes: in vitro solutions for cytotoxicity profiling, cell proliferation, signal transduction and vascularization assessment, biomarker identification and validation, and immune cells testing.
Our scientists have extensive expertise and know-how that can be applied to a wide range of pre-clinical and clinical research projects. We offer customized technical solutions that can best meet your requirements, prerequisites, and objectives, with particular attention given to the feasibility study and personalized management of your projects. With our state-of-the-art facilities and over 20 years of experience supporting projects in the pharmaceutical and biotechnological industries, we are pleased to offer you dedicated project management support and consulting for your R&D process.
*
Ready to discuss a testing and research program that will suit the needs of your business?