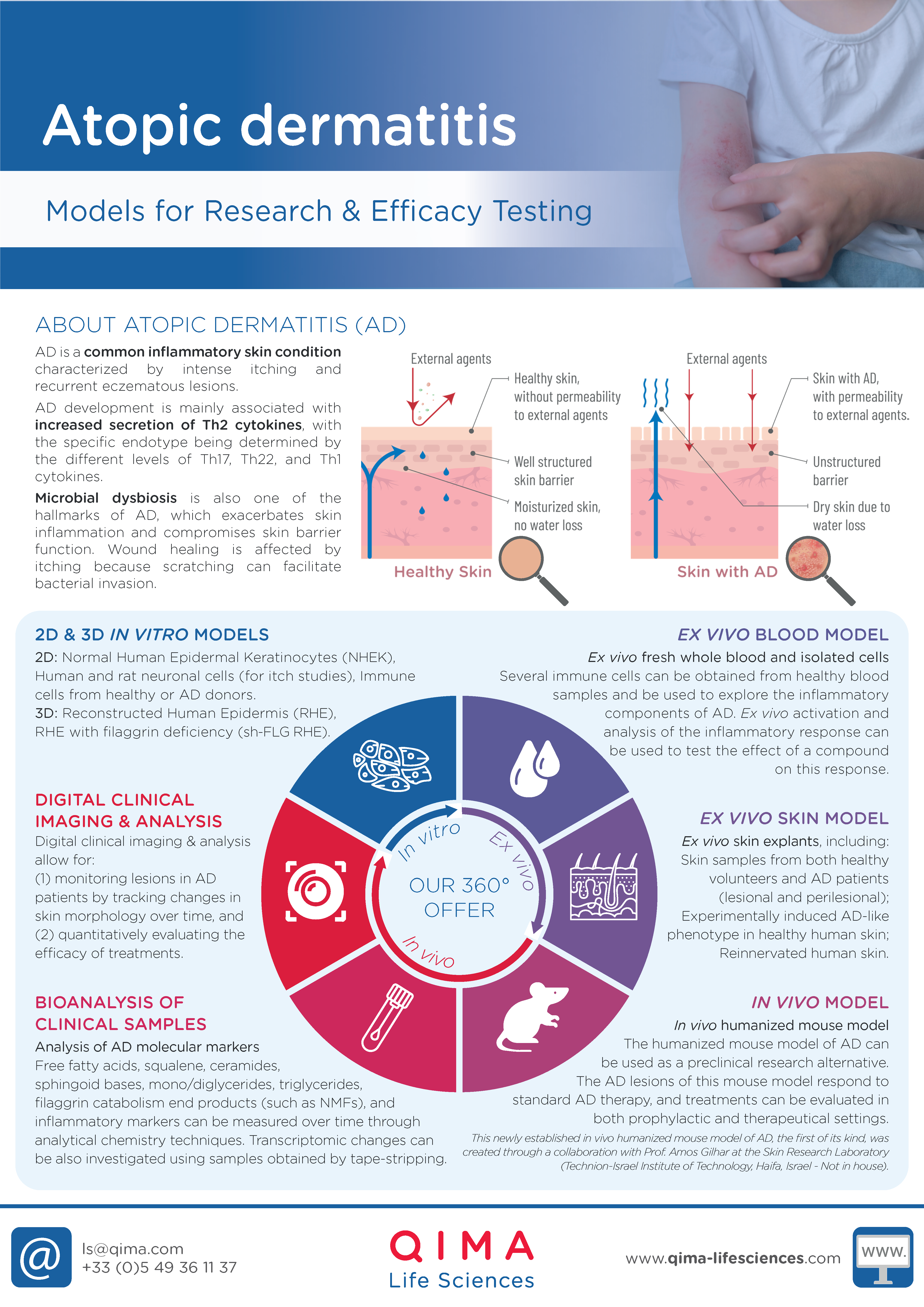
Atopic dermatitis, also known as eczema, is a chronic inflammatory skin condition that affects approximately 20% of children and up to 10% of adults in high-income countries.
It is characterized by epidermal barrier dysfunction and hypersensitivity to environmental factors. Symptoms include severe itching and recurring eczematous lesions, though the clinical presentation varies widely across different subtypes or endotypes.
The primary treatment for atopic dermatitis typically involves topical corticosteroids as the first line of therapy. More severe cases often require additional treatment options such as phototherapy, monoclonal antibodies, and JAK inhibitors.
Dermocosmetic topical products play a supportive role in treatment by strengthening the skin barrier and relieving symptoms. However, further research is needed to develop new treatments that are more effective, longer-lasting, have fewer side effects, and are safe for use in children.
At QIMA Life Sciences, we support our clients in drug discovery and development by providing advanced solutions for both preclinical and clinical stages.
Interested in learning how we can support your drug discovery and development?
Learn more below.
Preclinical Research Solutions for Atopic Dermatitis
IN VITRO MODELS
- NHEK (Normal Human Epidermal Keratinocytes)
- Experimentally-induced AD-like phenotype in NHEK
- Human and rat neuronal cells (for itch studies)
- Immune cells from healthy or AD patients
- RHE (Reconstructed Human Epidermis)
- Experimentally-induced AD-like phenotype in RHE
- RHE with filaggrin defficiency (sh-RHE)
EX VIVO MODELS
- Healthy human skin ex vivo
- Experimentally-induced AD-like phenotype in healthy human skin ex vivo
- AD-lesional and perilesional human skin ex vivo
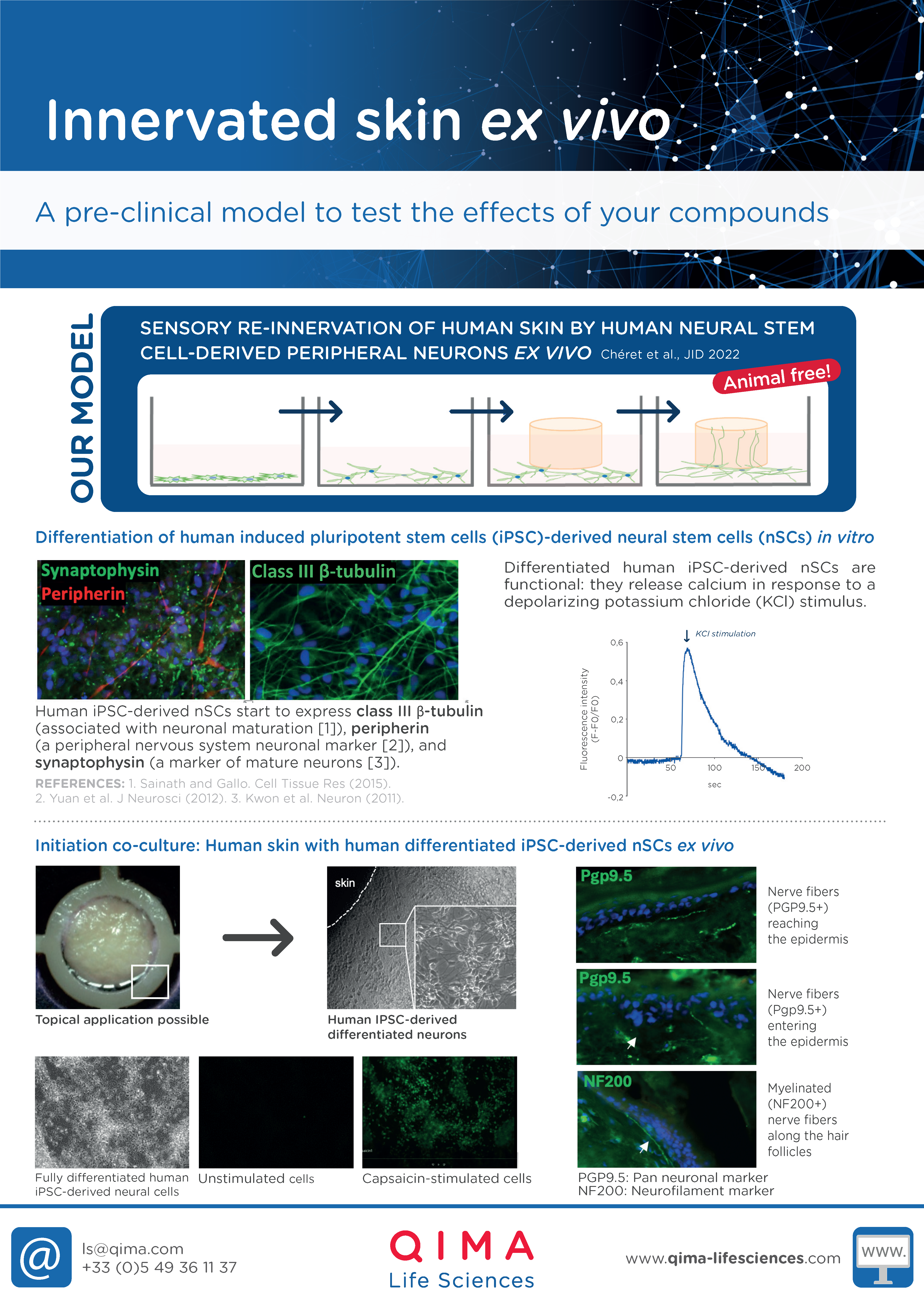
- Re-innervated human skin ex vivo (for itch studies)
- Fresh whole blood
IN VIVO MODELS
- In vivo humanized mouse model of AD (created through a collaboration with Prof. Amos Gilhar, Skin Research Laboratory, Rappaport Faculty of Medicine, Technion-Israel Institute of Technology, Haifa, Israel) Keren, A., et al. (2023). Allergy
Microbiome studies
Increasing evidence suggests that a reduced microbial diversity, with an overgrowth of Staphylococcus aureus on AD lesions, is linked to disease severity and skin barrier dysfunction. Moreover, in AD patients, intense itching often leads to scratching, which facilitates bacterial invasion and negatively impacts wound healing.
- To study these phenomena, S. aureus can be applied directly to reconstructed human epidermis (RHE) or human skin ex vivo. This allows for the examination of its impact on key factors and the investigation of wound healing processes in the context of AD.
Clinical Research Solutions for Atopic Dermatitis
BIOANALYSIS OF CLINICAL SAMPLES
- Non-invasive (swabbing or tape stripping) and invasive (biopsies) sample collection
- Analysis and quantification of cellular components (proteins, lipids) via analytical chemistry
- AD biomarker analysis in tissue, blood and non-invasive collected samples
CLINICAL IMAGING
- Image capture
- Image analysis of AD lesions (texture, roughness, redness, etc.)
- Grading solutions
CLINICAL TRIALS
- Clinical study implementation
- Clinical study performance
- Data management
- Data analysis
Atopic Dermatitis Study Examples
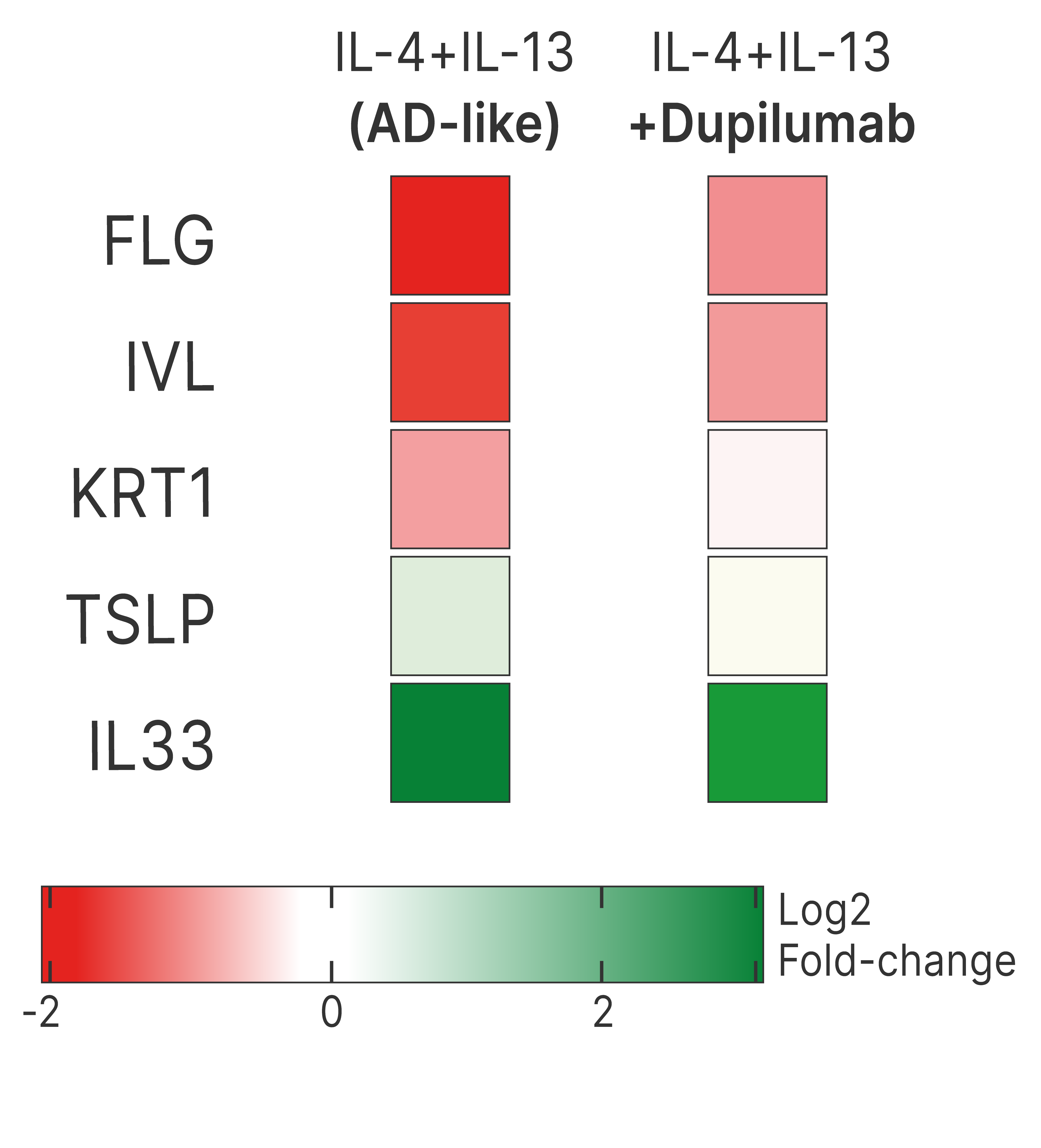
DUPILUMAB COUNTERACTS GENE EXPRESSION CHANGES INDUCED BY Th2 CYTOKINES IN KERATINOCYTES
Test: Transcriptomic analysis
Method: RNA seq
Model: Normal Human Epidermal Keratinocytes (NHEK)
Interpretation of results: Co-treatment with dupilumab counteracts IL-4 and IL-33-induced alterations in the expression of genes related to keratinocyte differentiation and skin barrier function (filaggrin, involucrin, and keratin 1), as well as those associated with type-2 immune responses (TSLP and IL-33).
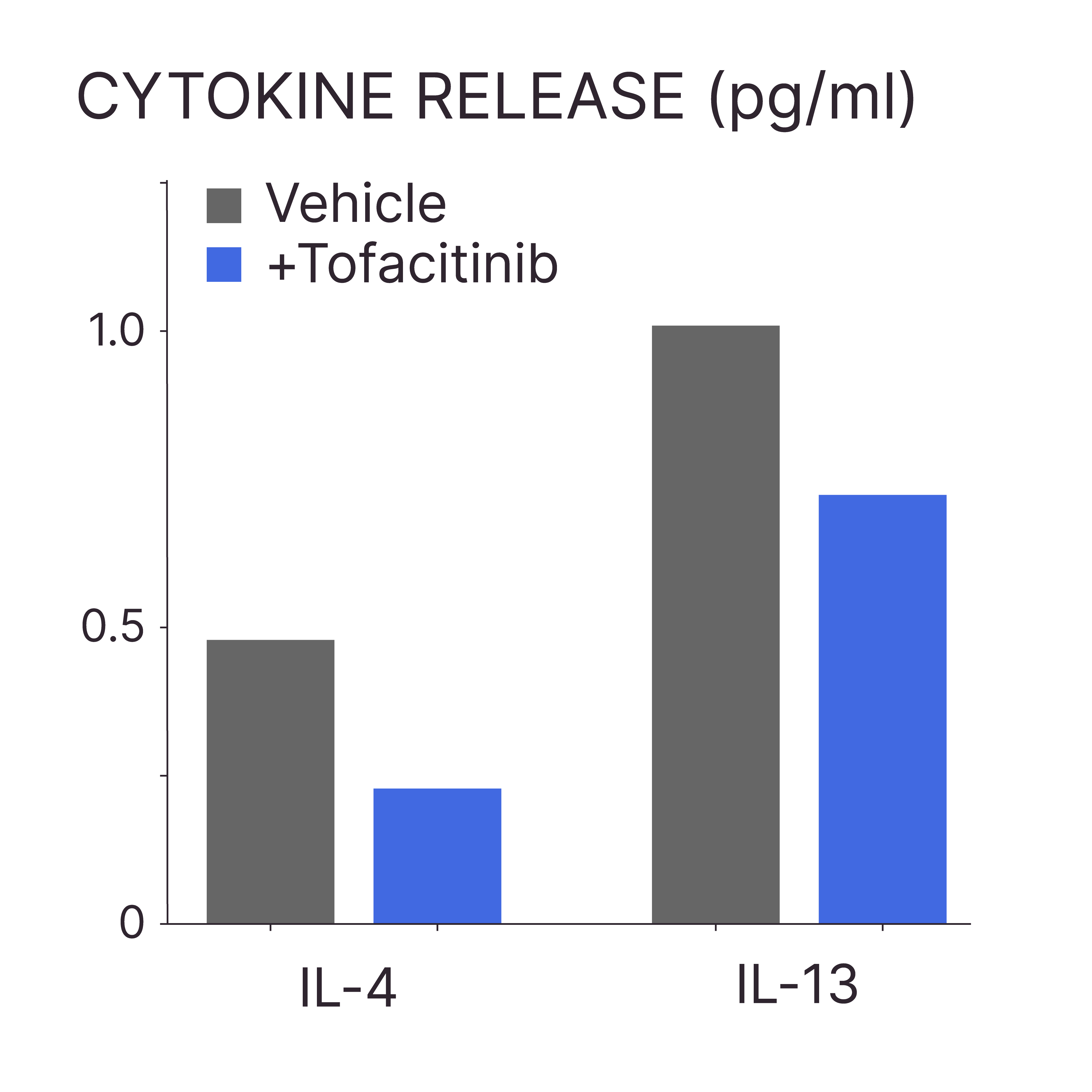
TOFACITINIB REDUCES THE RELEASE OF IL-4 AND IL-13 FROM AD-LESIONAL EX VIVO HUMAN SKIN
Test: AD-associated cytokine expression
Method: ELISA (or cytokine array)
Model: AD-lesional human skin
Interpretation of results: Tofacitinib treatment results in a decrease in the release of IL-4 and IL-13 into the culture medium.
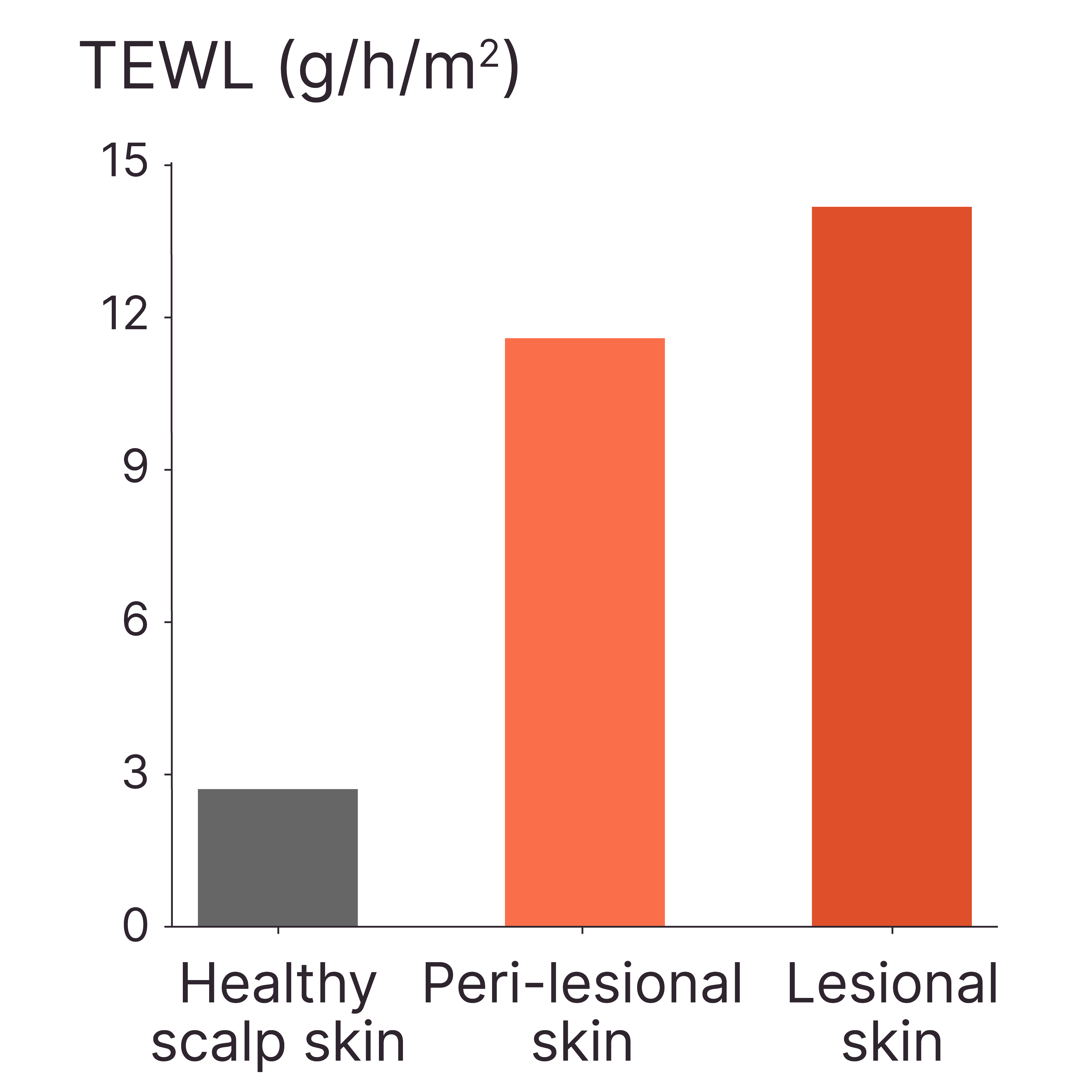
LESIONAL AD SKIN SHOWED HIGHER TEWL COMPARED TO PERI-LESIONAL SKIN AND HEALTHY SCALP SKIN
Method: Tewameter®
Model: Healthy and lesional AD skin ex vivo
Interpretation of results: The skin barrier function is impaired in both peri-lesional and lesional areas of patients with AD, with greater compromise observed in the lesional regions.
A PREBIOTIC CLEANSER AND MOISTURIZER ROUTINE EFFECTIVELY REDUCED SKIN ROUGHNESS IN AD LESIONS
Test: Lesion monitoring
Method: Image capture (SkinCam®) and analysis
Model: In vivo study on AD patients
Interpretation of results: After 10 weeks of using a prebiotic cleanser and moisturizer, volunteers with AD lesions experienced improvements in skin color texture contrast and skin texture irregularity.

At QIMA Life Sciences, we are committed to staying at the forefront of dermatology research by developing innovative approaches.
We offer smart solutions for studying atopic dermatitis using validated models at both preclinical and clinical stages, making us the perfect partner for your research.
Explore All Our Models & Assays in Our Brochure
Related Publications
What’s New in Testing?
PRECLINICAL SOLUTIONS
TEWL measurements on lesional and perilesional AD skin ex vivo
Re-innervated human skin ex vivo (for itch studies)
In vivo humanized mouse model of AD
CLINICAL SOLUTIONS
Image capture with SkinCam Pro® for monitoring of AD lesions and powerful image analysis to evaluate the efficacy of AD treatments in patients
Interested in Learning More?
Explore Other Related Topics
ATOPIC DERMATITIS
INNERVATED HUMAN SKIN EX VIVO
HIGH-DEFINITION IMAGING: SKINCAM PRO®

IMMUNODERMATOLOGY: ATOPIC DERMATITIS