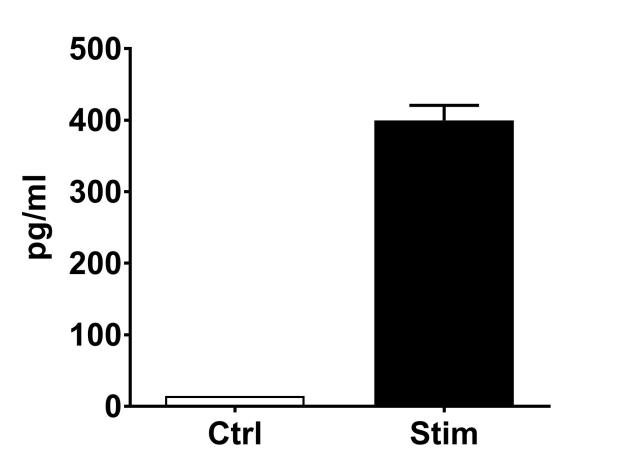
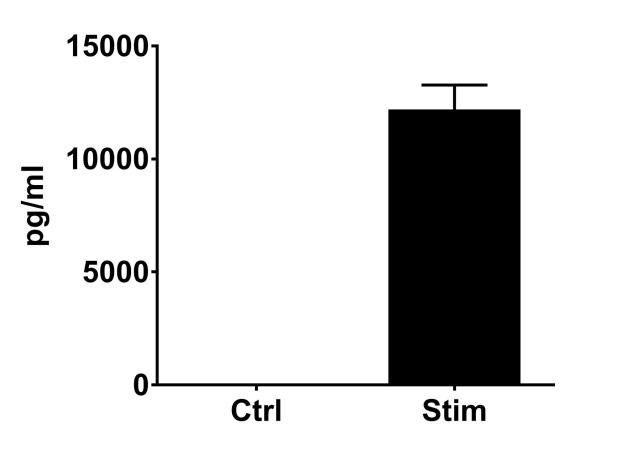
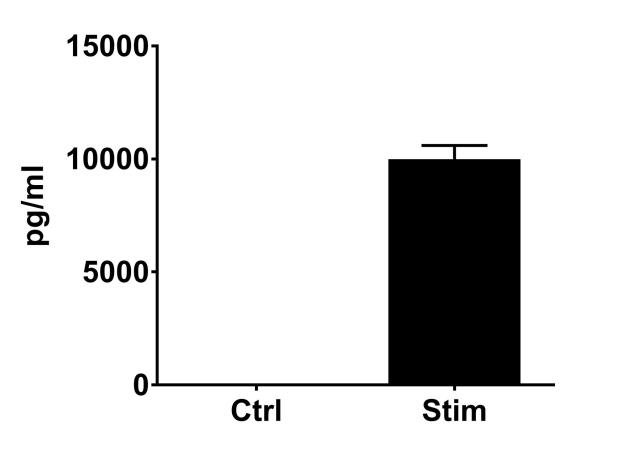
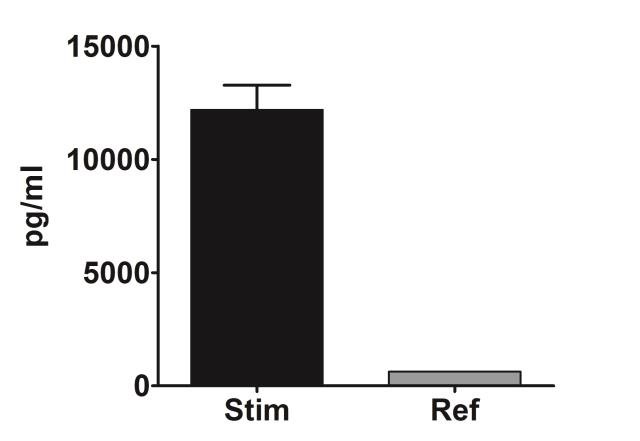
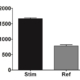
PBMC, anti-CD3/anti-CD28-stimulated T cells, Th1 cytokine release (anti-CD3/anti-CD28 stimulation)
PBMC-0008- Context
- Descriptions
- Requirement
- Study and reporting
- See also
| Biological Model | PBMCs |
|---|---|
| Species | Human |
| EndPoint | GM-CSF release |
| Method | ELISA / EIA |
| Stimulation Factor | Anti-CD3 (10 µg/ml) + Anti-CD28 (3 µg/ml) |
| Compound Reference | Cyclosporin A |
| Physiopathology | Blood and lymphatic system, cardiovascular system and immunity > Immuno-inflammation assays using blood/immune cells > PBMC |
Feasibility, study proposal:
Our sales team is here to help you find solutions:
Price: our pricing system consists of a fixed price per study + pricing per unit (price per replicate or experimental condition)
Start of assays and turnaround time: the start of an assay is subject to the receipt of your agreement and of the compounds to be tested. The sending date of the study report is communicated by email when the study starts. Although of an average of 5 to 10 weeks, our turnaround time may be subject to some fluctuation (due to availability of biological samples, to workload, etc.).
Required quantity of compounds: the required quantity is generally of a few mg or ml but may vary according to the form and solubility of compounds and to the concentrations to be tested. Please contact us for an estimation.
Customized protocols: for this assay, as for all of our assays, we can develop a protocol adapted to your needs. Should you have any question, please contact us.
Our sales team is here to help you find solutions:
- Technical feasibility study of your project
- Development of an assay selection strategy
- Definition of an experimental scheme
- Financial assessment and contractual agreements (costs, turnaround time, non-disclosure agreements, etc.)
Price: our pricing system consists of a fixed price per study + pricing per unit (price per replicate or experimental condition)
Start of assays and turnaround time: the start of an assay is subject to the receipt of your agreement and of the compounds to be tested. The sending date of the study report is communicated by email when the study starts. Although of an average of 5 to 10 weeks, our turnaround time may be subject to some fluctuation (due to availability of biological samples, to workload, etc.).
Required quantity of compounds: the required quantity is generally of a few mg or ml but may vary according to the form and solubility of compounds and to the concentrations to be tested. Please contact us for an estimation.
Customized protocols: for this assay, as for all of our assays, we can develop a protocol adapted to your needs. Should you have any question, please contact us.
Study management: each project requires advice and expertise so each study is managed and monitored by a dedicated study director who will be attentive to all your needs and questions.
Validation: the assays listed in the catalogue have undergone a preliminary validation and are subject to Standard Operating Procedures (SOPs) and to validation criteria. However, the robustness of each assay may be different according to the models and to the analytical methods. Please do not hesitate to contact us for more information.
Report and experimental data: our services include the writing of a study report in English or in French. This scientific report is provided in a PDF or Microsoft® Office format.
Laboratory / Location: this assay (as all of our assays) will be carried out in our Gençay laboratory in France.
Validation: the assays listed in the catalogue have undergone a preliminary validation and are subject to Standard Operating Procedures (SOPs) and to validation criteria. However, the robustness of each assay may be different according to the models and to the analytical methods. Please do not hesitate to contact us for more information.
Report and experimental data: our services include the writing of a study report in English or in French. This scientific report is provided in a PDF or Microsoft® Office format.
Laboratory / Location: this assay (as all of our assays) will be carried out in our Gençay laboratory in France.