NHEM, inhibition of melanin synthesis (L-tyrosine-induced, whitening agents)
NHEM-0018- Context
- Descriptions
- Requirement
- Study and reporting
- See also
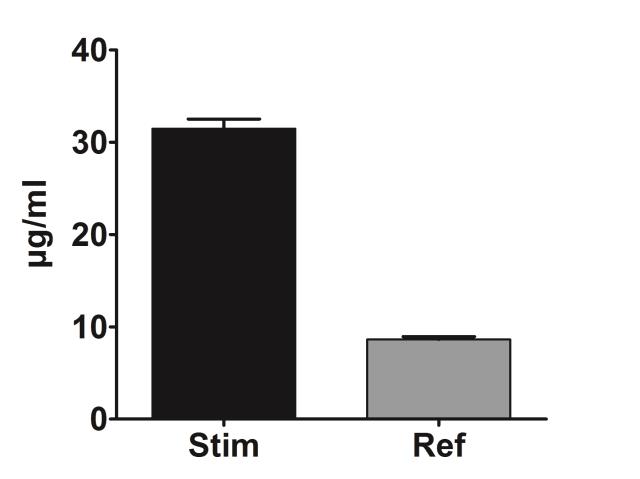
Human skin pigmentation is caused by the synthesis of melanin (a natural pigment) and by its distribution in skin and in hair follicles. Melanin synthesis, also called melanogenesis, is an enzymatic process that is mainly catalyzed by tyrosinase, tyrosinase-related protein 1 (TYRP1) and dopachrome tautomerase (DCT). These enzymes allow the biotransformation of tyrosine into melanin within melanocytes, and more particularly in melanosomes, which are lysosomal vesicles. Melanin plays an essential role in skin tone evenness and in photoprotection against UV-induced DNA damages. Melanin production can be increased by some internal factors (inflammatory or hormonal reactions) or by external factors (sun exposure). This increased melanin production can cause pigmentation damages or hyperpigmentation marks (age spots, melasma, acne lesions, etc.), which require skin care treatment. The purpose of this assay is to evaluate, via photometric determination, the capacity of compounds (molecules, active ingredients, extracts, etc.) to inhibit melanin production in a model of human epidermal melanocytes stimulated by L-tyrosine.
| Biological Model | NHEM-LP (Lightly Pigmented Normal Human Epidermal Melanocyte) |
|---|---|
| Species | Human |
| EndPoint | Melanin content |
| Method | Photometry |
| Stimulation Factor | L-tyrosine (1 mM) |
| Compound Reference | Lipoic acid |
| Physiopathology | Skin and cutaneous appendices > Melanocyte, pigmentation > Melanogenesis |
Feasibility, study proposal:
Our sales team is here to help you find solutions:
Price: our pricing system consists of a fixed price per study + pricing per unit (price per replicate or experimental condition)
Start of assays and turnaround time: the start of an assay is subject to the receipt of your agreement and of the compounds to be tested. The sending date of the study report is communicated by email when the study starts. Although of an average of 5 to 10 weeks, our turnaround time may be subject to some fluctuation (due to availability of biological samples, to workload, etc.).
Required quantity of compounds: the required quantity is generally of a few mg or ml but may vary according to the form and solubility of compounds and to the concentrations to be tested. Please contact us for an estimation.
Customized protocols: for this assay, as for all of our assays, we can develop a protocol adapted to your needs. Should you have any question, please contact us.
Our sales team is here to help you find solutions:
- Technical feasibility study of your project
- Development of an assay selection strategy
- Definition of an experimental scheme
- Financial assessment and contractual agreements (costs, turnaround time, non-disclosure agreements, etc.)
Price: our pricing system consists of a fixed price per study + pricing per unit (price per replicate or experimental condition)
Start of assays and turnaround time: the start of an assay is subject to the receipt of your agreement and of the compounds to be tested. The sending date of the study report is communicated by email when the study starts. Although of an average of 5 to 10 weeks, our turnaround time may be subject to some fluctuation (due to availability of biological samples, to workload, etc.).
Required quantity of compounds: the required quantity is generally of a few mg or ml but may vary according to the form and solubility of compounds and to the concentrations to be tested. Please contact us for an estimation.
Customized protocols: for this assay, as for all of our assays, we can develop a protocol adapted to your needs. Should you have any question, please contact us.
Study management: each project requires advice and expertise so each study is managed and monitored by a dedicated study director who will be attentive to all your needs and questions.
Validation: the assays listed in the catalogue have undergone a preliminary validation and are subject to Standard Operating Procedures (SOPs) and to validation criteria. However, the robustness of each assay may be different according to the models and to the analytical methods. Please do not hesitate to contact us for more information.
Report and experimental data: our services include the writing of a study report in English or in French. This scientific report is provided in a PDF or Microsoft® Office format.
Laboratory / Location: this assay (as all of our assays) will be carried out in our Gençay laboratory in France.
Validation: the assays listed in the catalogue have undergone a preliminary validation and are subject to Standard Operating Procedures (SOPs) and to validation criteria. However, the robustness of each assay may be different according to the models and to the analytical methods. Please do not hesitate to contact us for more information.
Report and experimental data: our services include the writing of a study report in English or in French. This scientific report is provided in a PDF or Microsoft® Office format.
Laboratory / Location: this assay (as all of our assays) will be carried out in our Gençay laboratory in France.