Development of a new model of reconstructed aged skin useful to study anti-ageing effects of cosmetic compounds
IFSCC 2016, 29th congress, Orlando
Alain DEGUERCY, Ph. D.(1), Christine BARRAULT(1) Ph. D., Nathalie PEDRETTI(1), M.D., Julien GARNIER(1), M.D., Romuald VALLEE(2), M.D., and Pierre-Yves MORVAN(2),Ph. D.
(1) Bioalternatives, 1 bis rue des Plantes, 86160 Gençay, France,
(2) CODIF International, La Poultière, 35610 Roz-sur-Couesnon, France
INTRODUCTION
The development of new anti-ageing products needs performant in vitro models mimicking morphological changes and physiological modifications appearing during skin ageing. In order to have access to a simple model mimicking the epidermis ageing but in relation with a normal dermis, we have developed a new in vitro model of reconstructed skin comprising an aged epidermis covering a reconstructed dermis built with collagen and normal (young) fibroblasts.
MATERIAL & METHODS
Reconstructed epidermis: normal human epidermal keratinocytes (NHEK) from 4 donors aged 7 years, 7 years, 19 years and 20 years were cultured and used at passages 2, 3, 4, 5, 6 and 7 for epidermis reconstruction. Passage of cells induces ageing as described by Hayflick. After 5 days and 12 days of incubation, the structure of the tissues was examined microscopically after Hematoxylin-Eosin-Safran staining of slices. Gene expression was analyzed using RT-qPCR arrays (64 genes) dedicated to keratinocytes differentiation and physiology.
Epidermis permeability: 14C-caffein was topically applied onto the surface of RHE built with keratinocytes from donor K1059 at passages 2, 3, 4, 5, and 6 and after 5 days and 12 days of differentiation. Radioactivity in the culture medium under the tissues was measured every 30 min for 8 h.
Reconstructed skin: normal human dermal fibroblasts (NHDF) were mixed with a solution of collagen to produce lattices. After contraction of the lattices, keratinocytes of donor K1059 (aged 7 years) from passages 2, 3, 4, 5, 6 and 7 were seeded at the surface of the dermal equivalents and incubated for 5 days and 14 days, in absence (control) or presence of the tested cosmetic ingredients. the structure of the tissues was examined microscopically after Hematoxylin-Eosin-Safran staining of slices. Gene expression was
analyzed using Affymetrix U219 chips (full transcriptome analysis).
RESULTS
1. Reconstructed epidermis (Fig. 1 & 2)
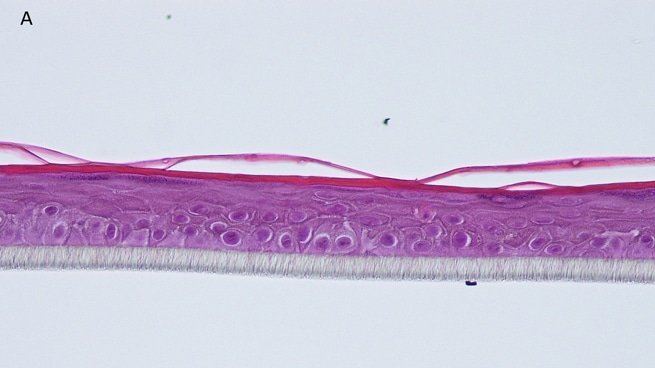
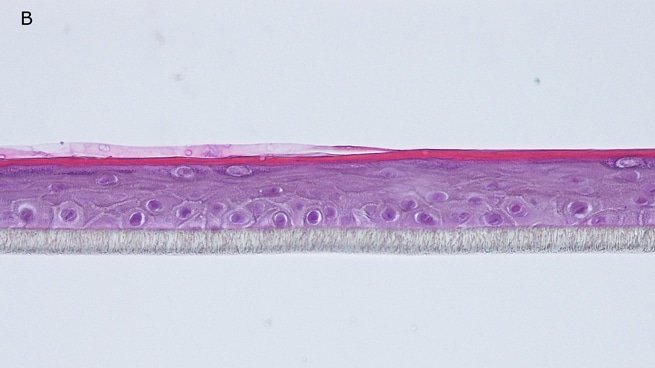
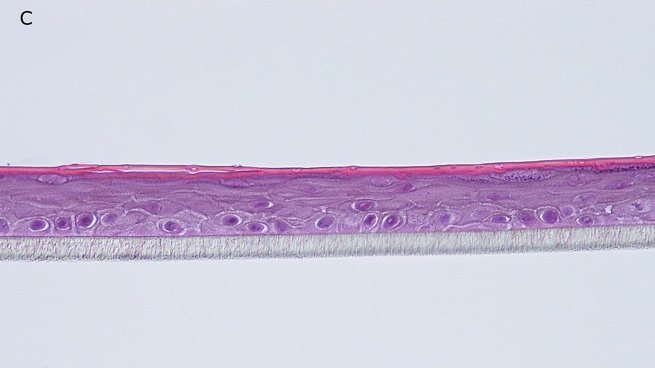
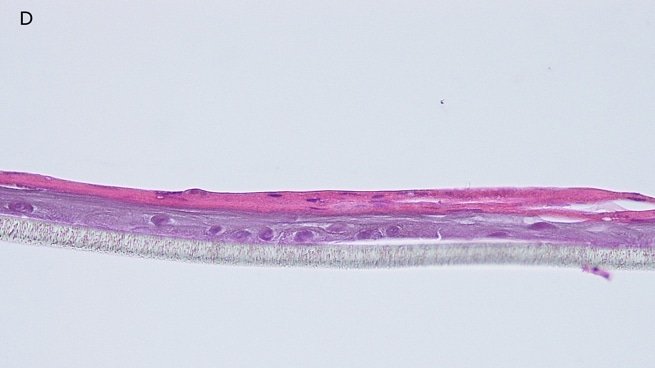
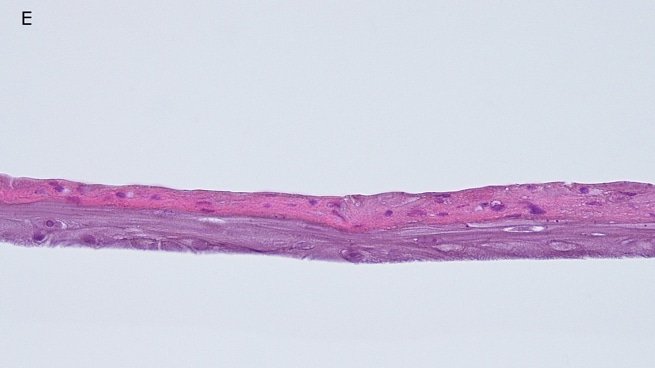
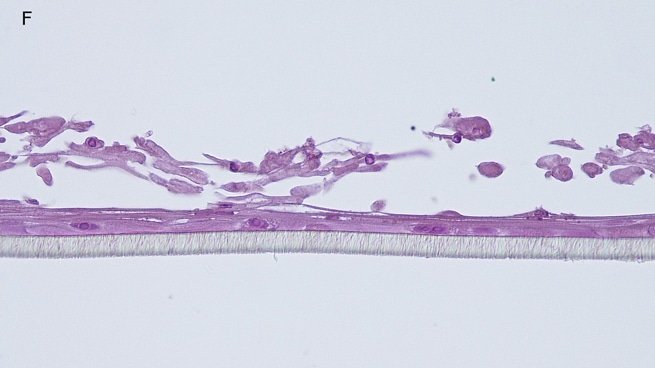
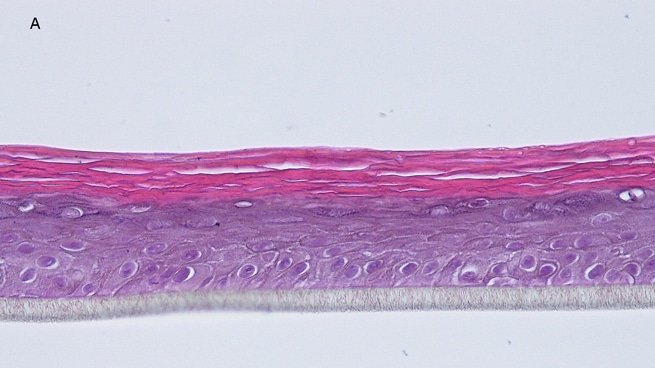
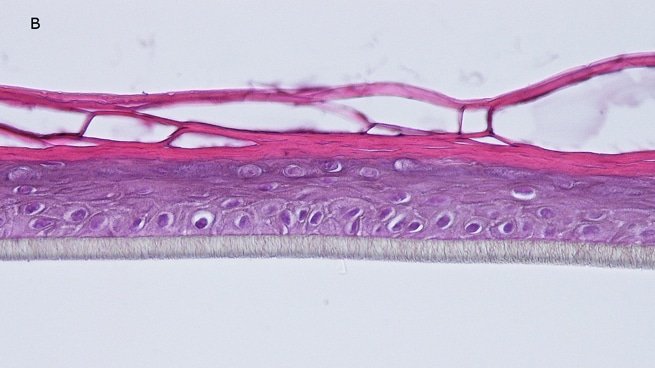
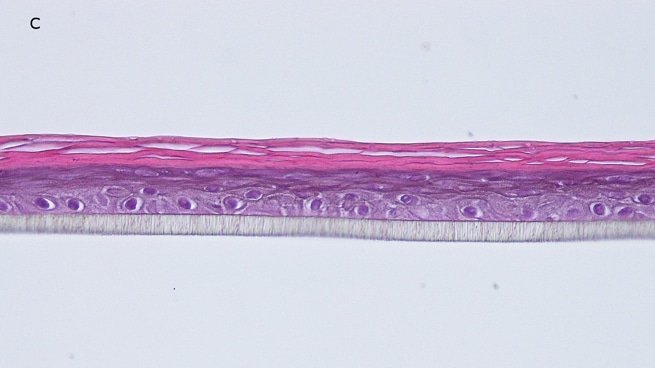
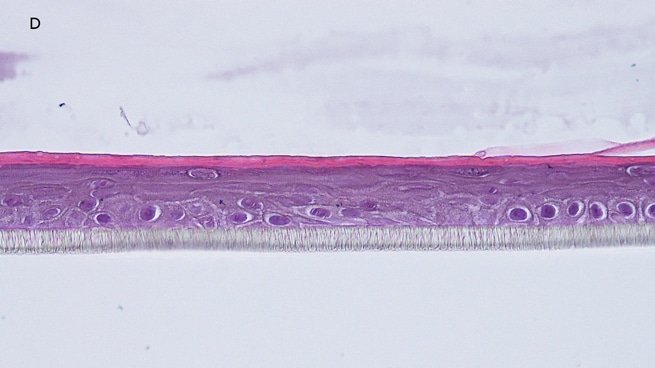
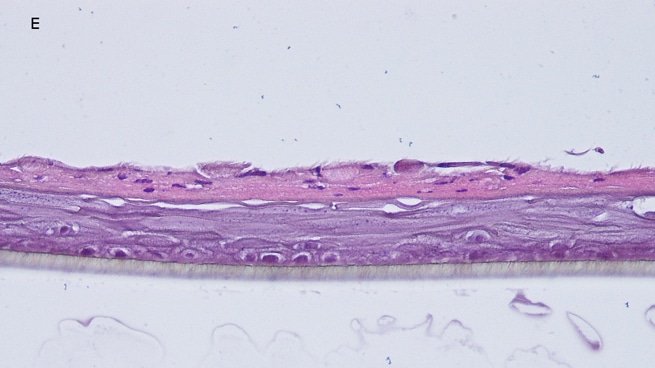
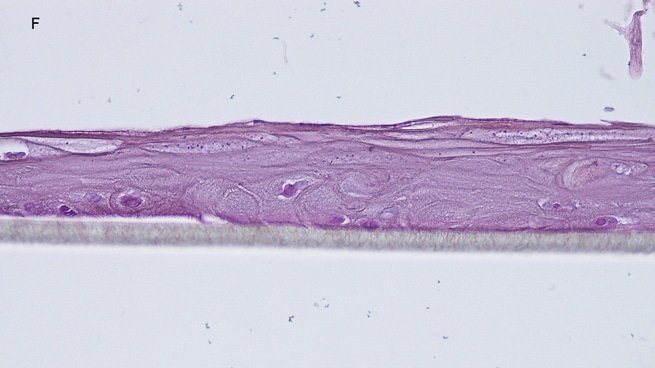
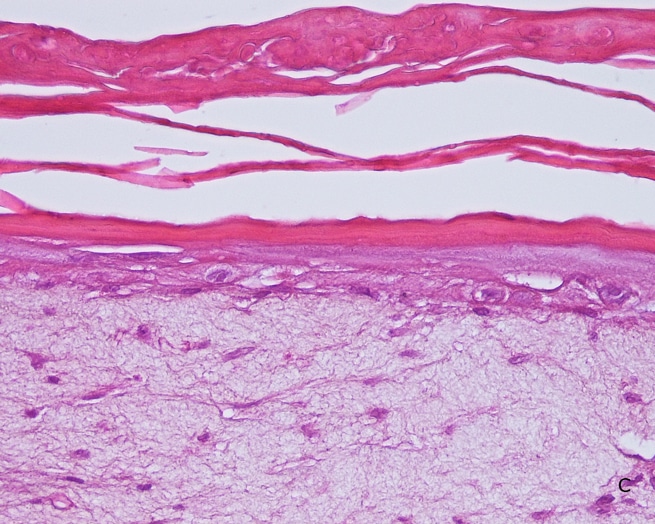
Histology: After incubation for 5 days and 12 days, reconstructed epidermis prepared with keratinocytes from passages 2 to 4 presented a normal organization. Those prepared with keratinocytes from passages 5, 6 and 7 presented abnormal differentiation, with parakeratosis and non differentiated layers (passage 7). Only tissues obtained with keratinocytes from donor K1059 (7 year old) are presented, but similar results were observed with keratinocytes from the other donors. Donor K1059 was selected for the other experiment because they presented a higher proliferation capacity.
Fig. 1: reconstructed epidermis produced with keratinocytes from donor K1059 (7 year old) after passages 2 (A), 3 (B), 4 (C), 5 (D),6 (E) and 7 (F), 5 days after seeding of cells
Fig. 2: reconstructed epidermis produced with keratinocytes from donor K1059 (7 year old) after passages 2 (A), 3 (B), 4 (C), 5 (D),6 (E) and 7 (F), 12 days after seeding of cells
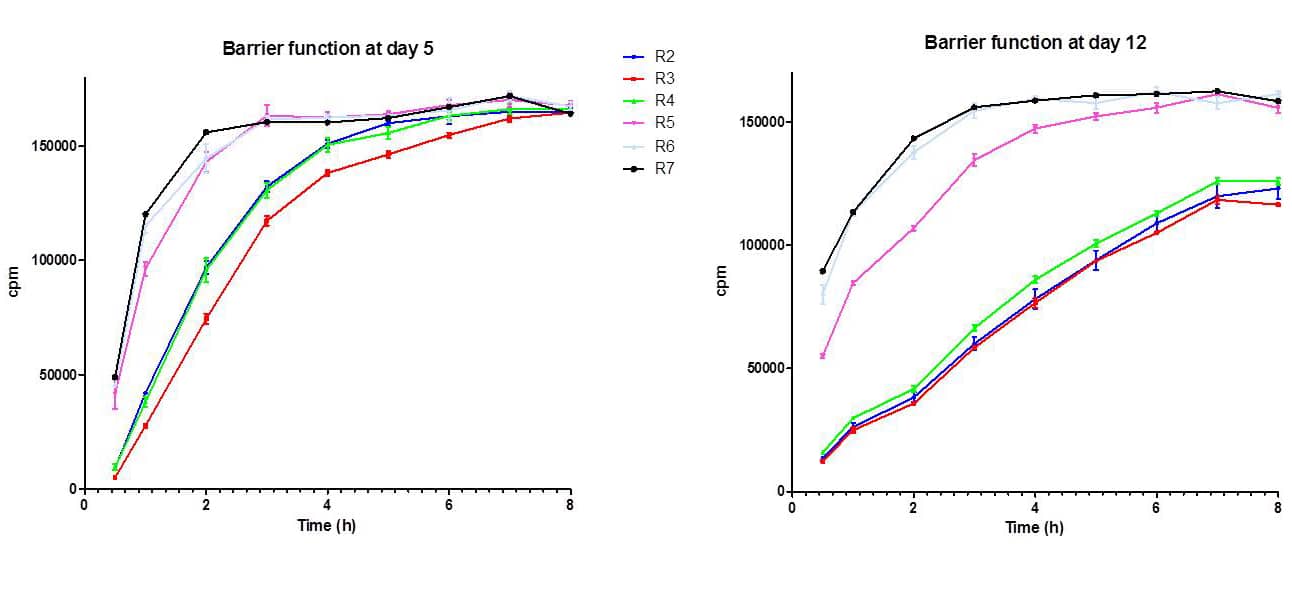
2. Epidermis permeability (Fig.3)
The rate of transepidermal passage of 14C-caffein was normal with P2, P3 and P4 reconstructed epidermis, whereas the permeability of P5, P6 and P7 epidermis was much higher , revealing an uncompleted differentiation of the tissue.
3. Gene expression in reconstructed epidermis (Table 1)
The higher passages showed a drastic decrease of the expression of genes involved in keratinocyte differentiation (calmodulin-like-5, caspase 14, filaggrin, keratin 1, sulfotansferase, etc.), in defenses (B4 defensin, S100A7 protein) and in cell-cell interactions (corneodesmosin, desmoglein). The expression of gene coding for keratin 19 and laminin gamma 2 was increased, mimicking a de-differentiation of keratinocytes. An inflammatory status is observed with the overexpression of interleukin 1a.
Fig. 3: barrier function at day 5 and day 12 in reconstructed epidermis prepared with keratinocytes at passages 2, 3, 4, 5, 6 and 7 (14C-caffein penetration)
Table 1: selection of the main genes showing a modulation in their expression in reconstructed epidermis prepared with keratinocytes from donor K1059 (7 year old) after passages 2 (A), 3 (B), 4 (C), 5 (D), 6 (E) and 7 (F), 12 days after seeding of cells.
4. Reconstructed skin (Fig.4)
Histology: After incubation for 5 days and 12 days, P5 and P6 reconstructed skin presented altered organization of cell layers in the epidermis, compared to P2 reconstructed skin (normal structure). The presence of normal (young fibroblasts) in the dermis didn’t normalize the differentiation of P5 and P6 epidermis.
5. Gene expression in reconstructed skin (Table 2)
The full genome Affymetrix analysis revealed that 1015 genes were upregulated (fold change >2) and 657 genes were down-regulated (fold change < 0.5) in P5 reconstructed skin vs. P2 reconstructed skin at day 14. Drastic modulation of genes involved in keratinocytes differentiation, cell-cell interaction, cell cycle regulation, matrix synthesis and degradation and inflammation confirmed and explain the histological abnormalities observed.
Fig. 4: reconstructed skin produced with normal dermal fibroblasts and keratinocytes from donor K1059 (7 year old) after passages 2 (A), 5 (B), and 6 (C), 14 days after seeding of cells
Table 2: selection of 53 genes having high modulation in their expression (P5 reconstructed skin compared to P2 reconstructed skin: mean of 3 experiments), after incubation for 12 days
6. Effects of compounds on “aged” reconstructed skin
Fifteen plant extracts and exopolysaccharides from marine microorganisms were tested in the culture medium of P5 reconstructed skin and compared to untreated P2 reconstructed skin. Amongst these compounds, EPS 5H DP4 tested at 0.0025 % (w/v) revealed spectacular reversions of gene expression modulation. The expression of 179 genes downregulated by ageing was increased by the treatment and the expression of 147 genes upregulated by ageing was decreased by the treatment. Mainly, EPS 5H DP4 stimulated epidermal differentiation, regulated cell proliferation and decreased inflammation induced by ageing.
CONCLUSION
The reconstructed skin model developed in this study can be a tool with the features of an aged skin epidermis. These reconstructed tissues can be used for evaluation of the remedial effects of ingredients and cosmetic formulations. The advantage of this model lies in the quality control of the tissues « older » and is not dependent on donors variability usually observed in skin explants used ex vivo.
Related posts
Check out Bioalternatives’ updates and experience new testing ideas
- Bioassays, models and services
- Posts and publications
- Events